Figure 3.
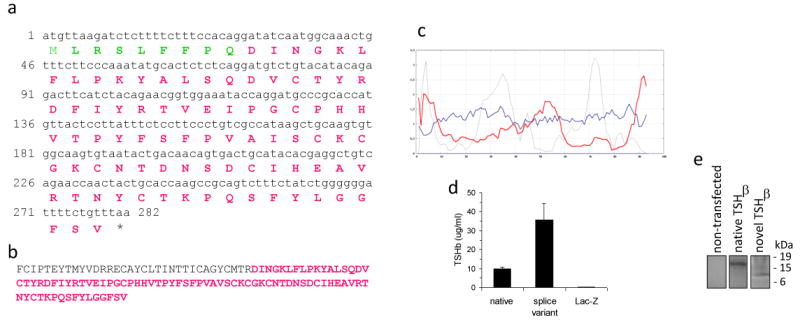
Amino acid composition of the novel TSHβ splice variant. (a) Predicted amino acid sequence of the novel TSHβ slice variant, consisting of a nine amino acid signal peptide (green residues) and an eighty-four amino acid polypeptide (red residues). (b) Location of the novel TSHβ isoform (red residues) within the 118 amino acid sequence of the mature native TSHβ molecule. (c) Secondary structure analysis of the novel TSHβ polypeptide. The grey line is the hydrophobic momentum index; the red line is the transmembrane helix momentum; the blue line is the beta preference index. Note the high hydrophobic momentum index and the high transmembrane helix momentum of the first 7-9 amino acids that would comprise the signal peptide. (d) TSHβ is secreted into the media from CHO cells transfected with native or splice variant TSHβ constructs, indicating that both forms of TSHβ are produced as secreted proteins. Control CHO cells transfected with LacZ had no detectable TSHβ. Data are mean values ± SEM of three replicate samples. (e) Cell lysates from non-transfected CHO cells were non-reactive by immunoprecipitation. Immunoprecipitation of cell lysates from CHO cells transfected with the native TSHβ construct produced a 17 kDa product; a 8 kDa product was precipitated from lysates of CHO cells transfected with the novel TSHβ construct.
