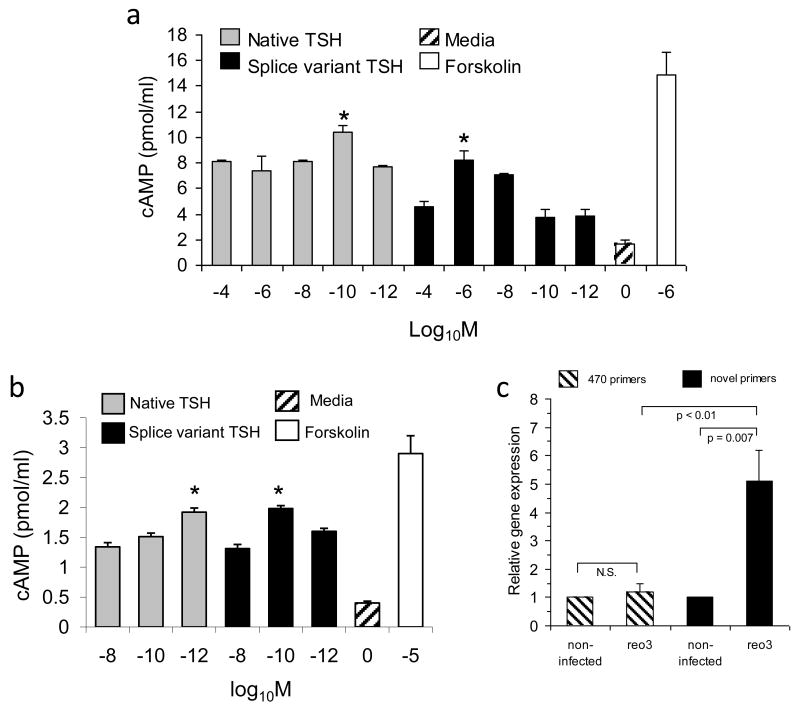
Figure 4.
Recombinant novel TSHβ splice variant is capable of delivering a cAMP signal and is upregulated in the thyroid following systemic virus infection. (a) cAMP response of AM cells cultured with log10 dilutions of recombinant native TSHβ, splice variant TSHβ, media, or forskolin at the concentration indicated. Both forms of TSHb elicited a cAMP response in a dose-dependent fashion. (*p<0.05 compared to other molar concentrations for that form of TSH). Data are mean values ± SEM of four replicate samples. (b) FRTL-5 cells, seeded into 24 well plates as described in the Material and Methods, were cultured with log10 dilutions of recombinant native TSHβ, splice variant TSHβ, media, or forskolin at the concentrations indicated. Both forms of TSHβ elicited a cAMP response in a dose-dependent fashion. (*p<0.01 compared to other molar concentrations for that form of TSH). Data are mean values ± SEM of three replicate samples. (c) qRT-PCR analysis of RNA from thyroid tissues 48 hrs post-reovirus infection using the 470 and novel primer sets. Note the statistically-significant increase in the novel TSHβ splice variant gene expression in the thyroid of infected mice compared to non-infected mice, and the lack of change in the thyroid in gene expression of native TSHβ in the thyroid during virus infection as identified with the 470 primers. Data are mean values ± SEM of three replicate values. In each case, gene expression of virus infected mice was compared to that of non-infected mice, the latter being designated as a gene expression level of 1.0.