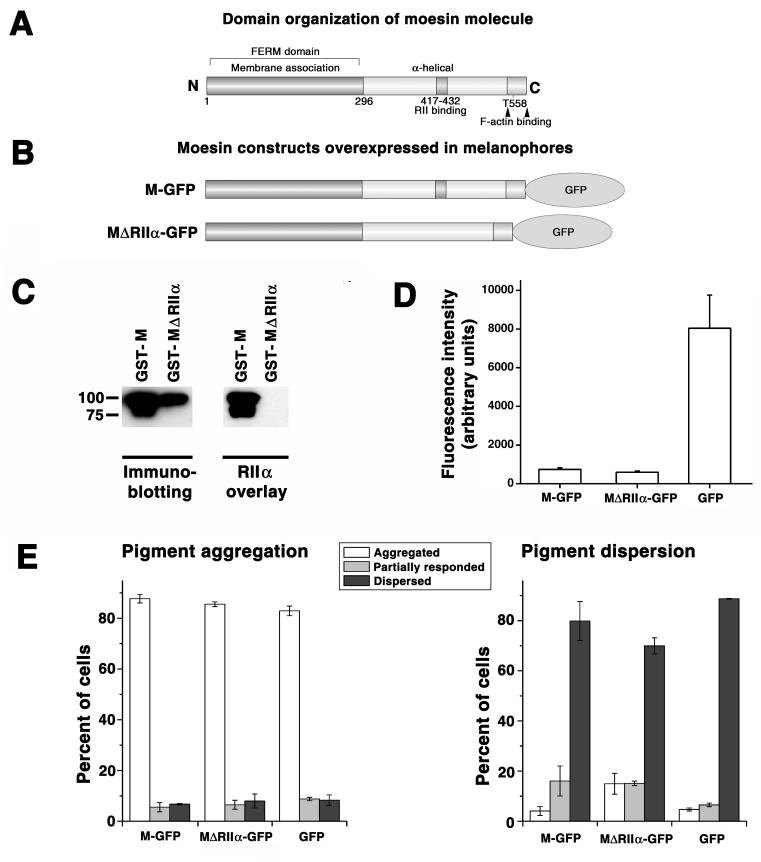
Figure 4. Overexpression of moesin lacking the RIIα-binding domain partially inhibits pigment dispersion.
A, domain organization of the Xenopus moesin molecule; the conserved RIIα binding domain (28) is located between amino acid residues 417 and 432. B, domain organization of the GFP-tagged Xenopus moesin constructs used for transfection of melanophores. C, blot overlay of the full-size recombinant GST-moesin and a mutant recombinant GST-moesin lacking the RIIα-binding domain with recombinant RIIα subunit of PKA; left panel, immunoblotting of purified recombinant proteins with moesin antibody; right panel, blot overlay with recombinant RIIα ; left lanes, full-size GST-moesin (GST-M); right lanes, GST-moesin lacking RIIα-binding domain (GST-MΔRIIα). Full-size recombinant moesin but not recombinant moesin lacking the RIIα-binding domain binds RIIα subunit of PKA. D, comparison of the levels of expression of GFP and moesin-GFP constructs shown in panel B in melanophores. Full-size moesin-GFP and a mutant moesin lacking RIIα-binding domain are expressed at approximately the same levels, whereas GFP is expressed at significantly higher levels compared to moesin constructs. E, Quantification of responses to melatonin or MSH, applied to induce pigment aggregation or dispersion, of melanophores overexpressing full-size moesin-GFP (left set of bars; M-GFP), moesin-GFP lacking conserved RIIa-binding domain (middle sets of bars; MΔRIIα-GFP), or GFP (right sets of bars; GFP); data are expressed as the percentage of cells with aggregated (white bars), partially dispersed (gray bars), or dispersed (black bars) pigment; left panel, stimulation of pigment aggregation with melatonin; right panel, stimulation of pigment dispersion with MSH. Overexpression of moesin constructs does not affect aggregation but partially inhibits dispersion, and inhibition is significantly more prominent in cells overexpressing moesin lacking the RIIα- binding domain (MΔRIIα-GFP) compared to full-size moesin (M-GFP).