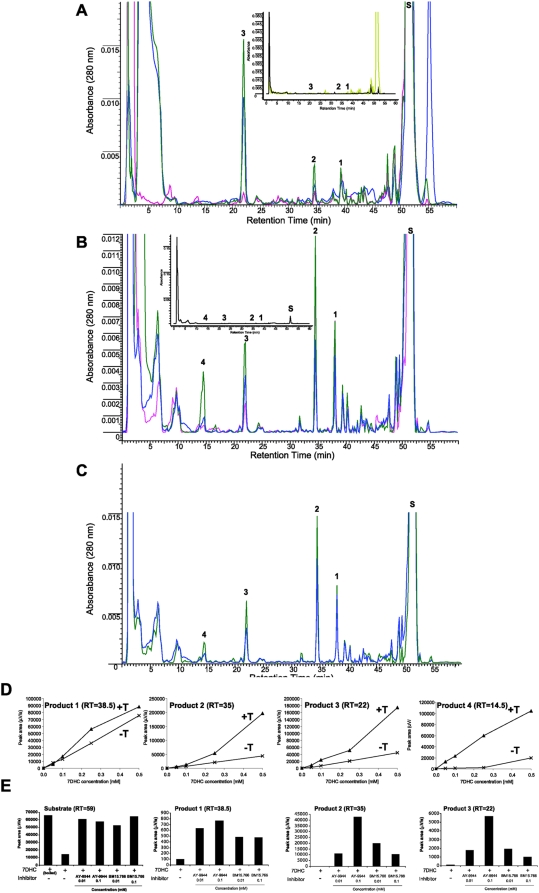
Figure 5. Metabolism of 7DHC by adrenal glands in the presence of DL-aminoglutethimide (AGT), trilostane, forscolin and Δ7-reductase inhibitors.
A. For rat adrenals transformation of 7DHC (S) to 7DHP (3) was dramatically inhibited by AGT (red) compared to the incubation with 7DHC alone (blue), while accumulation of 22(OH)7DHC (1), 20,22(OH)27DHC (2) and 7DHP (3) was enhanced by trilostane (green). Control incubations performed with boiled adrenal fragments are shown in the inset. Incubations were carried out for 18 h. B. For pig adrenals AGT also inhibited 7DHC metabolism (red) compared to the incubation with 7DHC alone (blue), while trilostane (green) enhanced accumulation of 22(OH)7DHC (1), 20,22(OH)27DHC (2), 7DHP (3) and 17(OH)7DHP (4). Adrenal fragments were incubated for 6 h and controls (inset) were incubated without the substrate. C. For pig adrenals 0.1 mM forscolin (green) stimulated 7DHC metabolism. The labeling and the experimental conditions are the same as in B. D. Fragments of pig adrenal glands were incubated with increasing doses of 7DHC in the presence (T+) or absence (T−) of 0.1 mM trilostane. The chromatograms were processed as described above. Trilostane (T) enhanced accumulation of 22(OH)7DHC (1), 20,22(OH)27DHC (2), 7DHP (3) and 17(OH)7DHP (4) as a function of 7DHC concentration. E. Fragments of rat adrenal glands were incubated with 7DHC (0.5 mM) in presence of AY-9944 or BM15.766 (10 µM or 100 µM) and analyzed by RP-HPLC (see A for peak identification). The chromatograms were processed using ACDLabs software and relative areas for peaks identified above were calculated. Δ7-reductase inhibitors enhanced production of 7DHC metabolites.