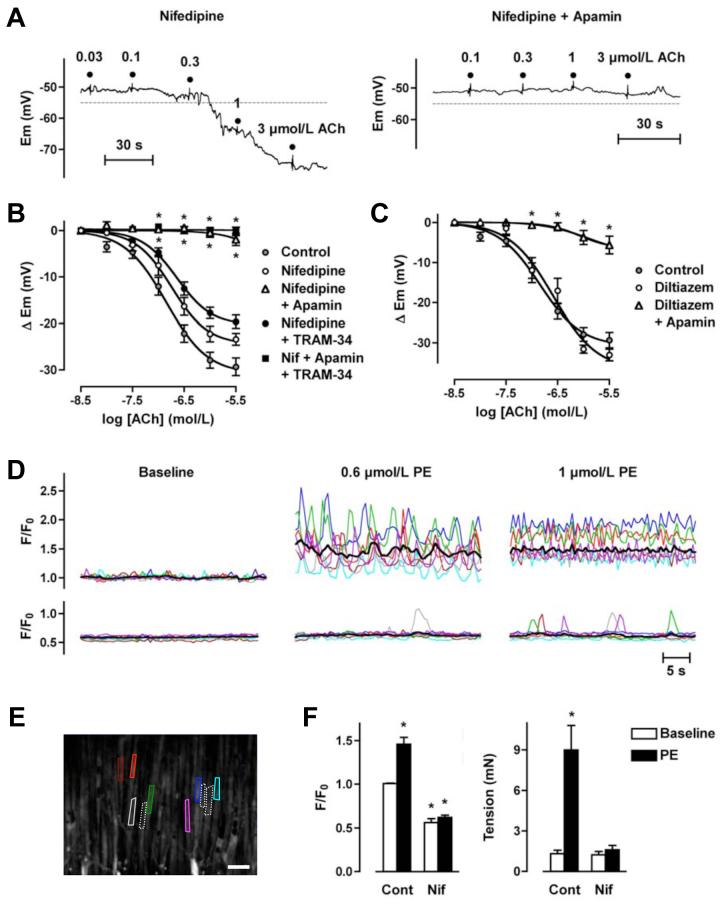
Figure 2.
ACh-mediated stimulation of KCa3.1-channels is prevented by blocking VGCC in rat mesenteric arteries. A, Original traces demonstrating concentration-dependent EDHF-induced smooth muscle hyperpolarization evoked by ACh in the presence of nifedipine (10 μmol/L) and prior depolarization to PE. Hyperpolarization overshot the resting membrane potential (dashed line), and was abolished by apamin (50 nmol/L). B, Summarized data showing the average change in membrane potential (Δ Em) to cumulative increases in [ACh] in arteries stimulated with PE in the presence of nifedipine (10 μmol/L, n=4-10), or in the additional presence of either apamin (50 nmol/L, n=4-12) or TRAM-34 (1 μmol/L, n=7-14), or both inhibitors together (n=5-8). C, Summarized data showing the average change in membrane potential (Δ Em) to cumulative increases in [ACh] in arteries stimulated with PE in the presence of diltiazem (10 μmol/L, n=4-6), or in the additional presence of apamin (50 nmol/L, n=5-7). D, Smooth muscle Ca2+ signals in the absence (upper traces) and presence of nifedipine (10 μmol/L, lower traces) colour-coded to the field of interest shown in the image in E. Applying 0.6 or 1 μmol/L PE stimulated a marked increase in the global average level of [Ca2+] (black line), representing asynchronous Ca2+ increases within individual muscle cells. Both the average increase and the majority of the oscillations were abolished in the presence of nifedipine (see Online Video 1 for movie corresponding to these data). F, Summarized data showing inhibition of the increase in [Ca2+]i stimulated by 1 μmol/L PE (left panel, control n=6, nifedipine n=3) and the associated block of arterial contraction (right panel, paired data). Asterisk indicates significant difference relative to control baseline (P<0.05).