Abstract
The vestibuloocular reflex (VOR) generates compensatory eye movements to stabilize visual images on the retina during head movements. The amplitude of the reflex is calibrated continuously throughout life and undergoes adaptation, also called motor learning, when head movements are persistently associated with image motion. Although the floccular-complex of the cerebellum is necessary for VOR adaptation, it is not known whether this function is localized in its anterior or posterior portions, which comprise the ventral paraflocculus and flocculus, respectively. The present paper reports the effects of partial lesions of the floccular-complex in five macaque monkeys, made either surgically or with stereotaxic injection of 3-nitropropionic acid (3-NP). Before and after the lesions, smooth pursuit eye movements were tested during sinusoidal and step-ramp target motion. Cancellation of the VOR was tested by moving a target exactly with the monkey during sinusoidal head rotation. The control VOR was tested during sinusoidal head rotation in the dark and during 30°/s pulses of head velocity. VOR adaptation was studied by having the monkeys wear ×2 or ×0.25 optics for 4–7 days. In two monkeys, bilateral lesions removed all of the flocculus except for parts of folia 1 and 2 but did not produce any deficits in smooth pursuit, VOR adaptation, or VOR cancellation. We conclude that the flocculus alone probably is not necessary for either pursuit or VOR learning. In two monkeys, unilateral lesions including a large fraction of the ventral paraflocculus produced small deficits in horizontal and vertical smooth pursuit, and mild impairments of VOR adaptation and VOR cancellation. We conclude that the ventral paraflocculus contributes to both behaviors. In one monkey, a bilateral lesion of the flocculus and ventral paraflocculus produced severe deficits smooth pursuit and VOR cancellation, and a complete loss of VOR adaptation. Considering all five cases together, there was a strong correlation between the size of the deficits in VOR learning and pursuit. We found the strongest correlation between the behavior deficits and the size of the lesion of the ventral paraflocculus, a weaker but significant correlation for the full floccular complex, and no correlation with the size of the lesion of the flocculus. We conclude that 1) lesions of the floccular complex cause linked deficits in smooth pursuit and VOR adaptation, and 2) the relevant portions of the structure are primarily in the ventral paraflocculus, although the flocculus may participate.
INTRODUCTION
As the head and body move, the vestibuloocular reflex (VOR) acts to stabilize images on the retina by generating compensatory smooth eye movements that are in the direction opposite to head motion. Under normal conditions, the ratio of eye to head speed in darkness (VOR gain) is close the ideal value of one (Fuchs and Kimm 1975; Keller 1978). Over the past 30 years, many studies have demonstrated that the VOR is subject to adaptive modification, also called motor learning. Several behavioral conditions induce adaptation, including viewing the world through miniaturizing or magnifying lenses, and visual-vestibular conflict presented during passive rotation on a turntable (e.g., Gonshor and Melvill Jones 1976; Ito et al. 1977; Miles and Fuller 1974).
Vestibular inputs for the VOR are transmitted to extraocular motoneurons through parallel pathways in the brain stem and cerebellum. Many of the neurons in these pathways have been identified physiologically and their activity recorded before and after VOR adaptation (Lisberger et al. 1994a,b; Miles et al. 1980). Available data on the effects of adaptation on both eye movements and neural responses can be reproduced by a model in which there are sites of adaptation in both the brain stem and cerebellar pathways. However, the model relies on assumptions regarding the identity and location of cerebellar Purkinje cells involved in adaptation. Specifically, the Purkinje cells in the successful model are based on the horizontal gaze velocity Purkinje cells (HGVPs) recorded from the floccular complex of the cerebellum. HGVPs show modulation of simple-spike firing during smooth pursuit eye movements in normal animals, and during the VOR after adaptation. The responses after VOR adaptation seem to reflect changes associated with learning and are in the correct direction to support the adapted VOR. The floccular complex, however, is anatomically complex and heterogeneous. It consists of four caudal lobules that comprise the flocculus, one that comprises a transition zone called the medial extension, and five or six rostral lobules that are part of a different and evolutionarily newer structure, the ventral paraflocculus. Nagao (1992) argues that the evolutionarily older flocculus is involved in VOR adaptation, whereas the evolutionarily newer ventral paraflocculus and the HGVPs participate in smooth pursuit eye movements, which seem to have evolved along with primates. Because the HGVPs that change following adaptation are distributed across the boundary between the flocculus and ventral paraflocculus, current data do not test the hypothesis of Nagao (1992).
The goal of our experiments was to determine whether smooth pursuit eye movements and VOR adaptation were mediated by separate parts of the floccular complex, namely the ventral paraflocculus and the flocculus, respectively. Prior lesion studies have not attempted to examine the differential contributions of the two subdivisions. Lesions of the entire cerebellum in monkeys abolished pursuit (Westheimer and Blair 1974). Bilateral, nearly complete lesions of the floccular complex in monkeys caused impairment of horizontal smooth pursuit (Zee et al. 1981) and adaptation of the horizontal VOR (Lisberger et al. 1984). Deficits in horizontal VOR adaptation were also caused by bilateral chemical destruction of the flocculus in rabbits (Nagao 1983), ablation of the flocculus in mice (Koekkoek et al. 1997), removal of the entire cerebellum in cats (Robinson 1976) and goldfish (Michnovicz and Bennett 1987), or microdialysis of lidocaine in the goldfish cerebellum (McElligott et al. 1998). Unilateral lesions of the floccular-complex in the rabbit cause deficits in adaptation of the VOR (Ito et al. 1982).
Our approach was to make partial lesions of the flocculus and ventral paraflocculus and determine whether deficits in pursuit and VOR learning could be dissociated. We show that there is a strong correlation between the size of the deficits in smooth pursuit and the VOR, arguing that the neurons supporting the two behaviors are distributed across similar regions of the floccular complex. We also show that there is a correlation between the size of the deficits and the size of the lesion of the ventral paraflocculus, but not the size of the lesion of the flocculus. Our data imply that the ventral paraflocculus plays an important role in both behaviors and that the flocculus, while it could participate, may not be necessary for either.
METHODS
Lesions of restricted portions of the floccular complex were made in five 4- to 9-kg macaque monkeys (4 rhesus, 1 fascicularis). Two monkeys received neurotoxin injections in the ventral paraflocculus, two received suction ablations of the flocculus, and one received a suction ablation of the flocculus followed by neurotoxin injections in the ventral paraflocculus. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were described in detail in a protocol that had been approved in advance by the Institutional Animal Care and Use Committee at UCSF.
Methods described in earlier papers (Lisberger and Westbrook 1985) were used to train the monkeys to track a moving spot. Monkeys were then anesthetized with isofluorane and implanted, under sterile surgical conditions, with bolts in the skull to restrain the head (Miles and Eighmy 1980), a scleral search coil for measuring horizontal and vertical eye position (Judge et al. 1980), and a stainless steel cylinder for introducing microelectrodes into the cerebellum and injecting neurotoxin. The cylinder was tilted 26° back from the coronal plane and was aimed 10 or 11 mm lateral to ear bar zero (Lisberger et al. 1994b). During experiments, monkeys sat in a specially designed primate chair, and their heads were restrained using the implanted bolts.
Data acquisition
Experiments were controlled and data acquired by a computer system that consisted of a UNIX workstation and a Pentium-based PC. Depending on the stimulus condition, signals related to horizontal and vertical eye and target position, and horizontal head position, eye velocity, and head velocity were digitized at a sampling rate of 500 or 1,000 samples/s per channel. Horizontal and vertical eye position signals came directly from the eye coil electronics. Eye velocity signals were obtained using an analog circuit (band-pass: DC to 25 Hz) to differentiate the eye position signals. Target position signals were derived from the feedback from mirror galvanometers that were used to control target position. Horizontal head position and velocity were obtained from a potentiometer and tachometer connected to the shaft of the turntable. A circular 0.5° pursuit target was created by reflecting the beam from a fiber-optic light source off an orthogonally placed x–y pair of mirror galvanometers and projecting onto the back of a tangent screen. The galvanometers were driven by the D/A outputs from a Pentium PC computer.
VOR adaptation
We induced changes in the gain of the VOR by fitting monkeys with lenses (Lisberger and Pavelko 1986; http://keck.ucsf.edu/~sgl/top_goggles.htm) to miniaturize (by a factor of 0.25) or magnify (by a factor of 2.2) the visual scene. The spectacle frames were constructed from dental acrylic and were molded to each monkey’s face individually. The frame was secured to the head-post, and the optics were centered on the monkey’s eyes. Monkeys wore the goggles in their home cages and underwent VOR adaptation during the visual-vestibular interaction created by their active head turns. A full adaptation cycle comprised 4–7 days of experience with one set of goggles, 2–3 days for the VOR to return to normal, and 4–7 days of experience with the other set of goggles. The fitting of the goggles was checked and the VOR in the dark was tested daily, at approximately the same time every day to ensure that we obtained measurements at 24-h intervals.
The VOR was tested during pulses of head angular velocity in the dark provided by a servo-controlled 20-ft-lb rotator. Each pulse consisted of a rapid head acceleration from 0 to 30°/s in 50 ms, head movement at 30°/s for 200 ms, and a rapid deceleration back to 0°/s in 50 ms (Lisberger and Pavelko 1986). Pulses moved the head alternately leftward and rightward with an interval of 1.096 s from the start of one pulse to the start of the next, creating a trapezoid of head position. Monkeys were required to fixate a small stationary spot when the head was stationary. To ensure that the vestibular stimulus was presented in the darkness, the spot was extinguished whenever the head was being turned. This way, the gain of the VOR could be measured progressively over a week-long adaptation period in the absence of any paired visual-vestibular stimulation when the goggles were off. Data were analyzed by averaging eye velocity for 10 saccade-free pulses of head velocity in each direction. The gain of the VOR was estimated as eye speed divided by head speed during the second 100 ms of rotation at constant velocity.
We also tested the VOR during sinusoidal head movements. Sine waves were provided over the frequency range from 0.5 to 8 Hz with the amplitude adjusted so that the peak angular head velocity was 30°/s. Data were analyzed by using a cursor to point out the start and end of saccades so that the rapid deflections of eye velocity they caused could be replaced with line segments that connected smooth eye velocity before and after the saccade. We then averaged the responses to multiple cycles of the same frequency and subjected the head and eye velocity averages to Fourier analysis using the fast Fourier transform (FFT). The gain of the VOR was defined as the amplitude of the fundamental component of the eye velocity response divided by the amplitude of the head velocity stimulus.
Smooth pursuit eye movements
Pursuit was studied with step-ramp target motions like those described in earlier papers (Lisberger and Westbrook 1985; Rashbass 1961). To initiate a trial, the monkey had to fixate a stationary spot for a random interval that averaged about 500 ms. The target then stepped to an eccentric position that was customized to minimize the number of early saccades, and immediately began to move at a constant speed. In randomly interleaved trials, target speeds could be 10, 20, or 30°/s up, down, left, or right. We analyzed only the responses to target motion toward the position of fixation, for which there were few early saccades, but we included an equal number of trials in which target motion was away from the position of fixation. Step-ramp target motion was used to measure pursuit because it contains an open-loop interval in which the smooth eye motion is driven directly by visual inputs without feedback, and therefore is likely to be a sensitive indicator of the integrity of pursuit pathways. Twenty-four different target trajectories were randomly presented, making the task highly unpredictable. To analyze the data, we used a cursor to point out the start and end of the rapid deflections of eye velocity during saccades and replaced them with line segments connecting eye velocity before and after the saccade. We then averaged the eye velocity from at least 10 responses to the same target motion and used the averages to compute latency using the method of Carl and Gellman (1987), steady-state gain as the mean eye velocity in the interval from 100 to 250 ms after the onset of pursuit, and peak initial eye acceleration as k1 in the equation
where k1 is the maximal amplitude, k2 is the latency, and k3 is the time constant of rise of eye velocity (Ė[t]). Responses to sinusoidal target motion were analyzed in the way described for the VOR during sinusoidal head motion, except that target position was substituted for head velocity.
Cancellation of the VOR
Cancellation of the VOR was tested by oscillating the turntable sinusoidally and moving the target in phase with the turntable. Sine waves were provided over the frequency range from 0.5 to 8 Hz with the amplitude adjusted so that the peak angular head velocity was 30°/s. Data were averaged using the same methods as for sinusoidal VOR and pursuit and subjected to FFT. The gain of VOR cancellation was defined as
where Ė is the amplitude of the fundamental component of the eye velocity response and Ḣ is the amplitude of the head velocity stimulus.
Lesions and ablations
Bilateral surgical lesions of the flocculus were performed using a sub-occipital approach. A craniectomy allowed us to expose the sigmoid sinus laterally and the transverse sinus superiorly. The dura was opened and reflected over the sinuses. Gentle and progressive retraction of the cerebellar lobe exposed the cerebello-pontine angle. The dorsal paraflocculus and lobulus simplex were gently retracted exposing the flocculus, which was removed bilaterally by sub-pial suction. The vestibular nerve was used as a landmark for the rostral extent of the lesion. After careful hemostasis had been performed, the dura was sutured and the muscle and skin were closed. Monkeys were given an analgesic (Buprenorphine HCL, 0.01 mg/kg every 12 h) and were fed by hand until they were visibly thriving. This recovery period ranged from a few days to a few weeks. The ablations achieved by the surgical approach removed only the more caudal extent of the floccular complex: the flocculus only or the flocculus and most caudal parts of the ventral paraflocculus.
Irreversible chemical lesions of the ventral paraflocculus were created by injecting the neurotoxin 3-nitropropionic acid (3-NP) under stereotaxic control. 3-NP induces an acute excitotoxic necrosis and a delayed apoptosis (Pang and Geddes 1997) by inhibiting the enzyme succinate dehydrogenase and the tricarboxylic acid cycle in neurons and astrocytes (Olsen et al. 1999). We therefore waited 3 days following each injection into the cerebellum before evaluating the full behavioral deficit, as this is the reported necessary delay for the excitotoxic lesion to be completed (Chyi and Chang 1999). Pilot experiments in mice were used to determine the appropriate concentration, which was 60 µg/ml. We chose to use 3-NP instead of the more conventional excitotoxic substances after pilot experiments indicated that we would have more consistent success with the 3-NP.
Injection sites were selected after mapping the relevant area with electrical recordings. Recording electrodes approached the floccular-complex through the cerebral cortex and tentorium. Following a sharp increase in background activity from the entry into the cerebellar cortex, the location of the electrode in the floccular complex was confirmed by recording responses to eye movements (Lisberger and Fuchs 1978; Miles et al. 1980; Stone and Lisberger 1990). Injections were performed by replacing the recording electrode with injectrodes comprising a standard recording electrode cemented to a guide tube for the injection needle. The tip of the guide tube came within 1 mm of the tip of the electrode, allowing physiological control over the injection sites by enabling injections close to sites with activity related to eye movements. At these sites, a Hamilton syringe was lowered through the guide tube to the appropriate depth, and a volume of 1–2 µl of 3-NP (concentration: 60 µg/ml) was injected slowly over 10–15 min. Up to 10 injections were made on each side of the cerebellum, usually over a period of weeks.
After postoperative testing the monkeys were killed under deep pentobarbital anesthesia by intra-cardiac perfusion with buffered saline followed by 4% paraformaldehyde (pH 7.4). The cerebellum was blocked in the stereotaxic coronal plane and was then sent to Neuroscience Associates (Knoxville, TN) to be embedded, cut in 40-µM sections, and stained with thionin.
RESULTS
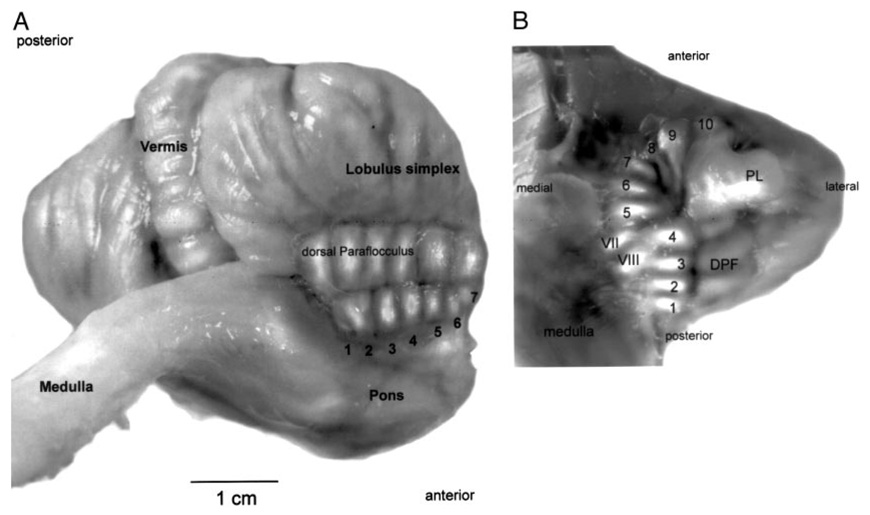
To allow comparison with experimental data from other reports, we first illustrate the normal anatomy and nomenclature of the 10 or more folia comprising the floccular complex (Fig. 1). The flocculus consists of folia 1–4 and the ventral paraflocculus of folia 6–10 or 11. The two structures are separated by a transition zone called the medial extension (ME), that we assign to folium 5 after Gerrits and Voogd (1989). For simplicity, however, we will consider folium 5 to be part of the ventral paraflocculus, as it lies just rostral to the postero-lateral fissure (PLF in Fig. 1B). The nearby petrosal lobule (PL in Fig. 1B) is easily identified in situ as it resides in a bony tube that is encircled by the superior semicircular canal.
FIG. 1.
Views of the floccular-complex of the monkey cerebellum from the postero-lateral side (A) and the ventral side (B). Numbered folia are part of the floccular complex. Folia 1–4 comprise the flocculus, while folia 5–10 comprise the ventral paraflocculus. PL, petrosal lobule; DPF, dorsal paraflocculus; PLF, postero-lateral fissure; VII, facial nerve; VIII, vestibulo-cochlear nerve.
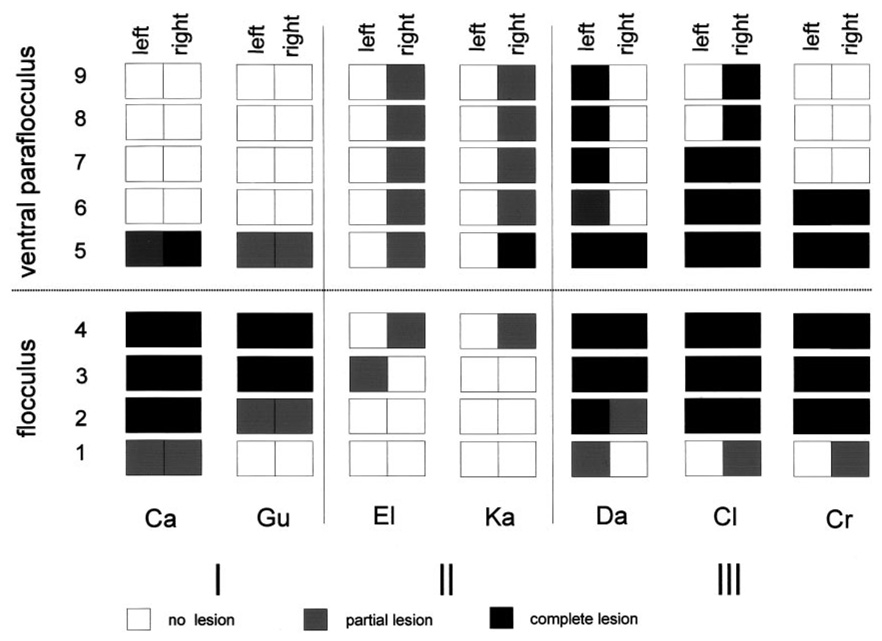
To compare the extent of the lesions in seven cerebella, two of which were previously reported by Lisberger et al. (1984) (monkeys Ca and Cr), we reconstructed the histology from coronal sections and scored each individual folium as intact, incomplete, or absent (white, gray, and black squares, respectively, on Fig. 2). In spite of the variation in the plane of sectioning and/or the individual anatomy, this method allowed side-by-side comparison of the floccular complex from different cerebella as illustrated in Fig. 2. We include the histological data from the two monkeys of Lisberger et al. (1984) to place our results in the context of the full set of available floccular lesion data on VOR adaptation in monkeys, but we will not repeat the earlier report of the physiological data from these two monkeys. We did not attempt to reconstruct the extent of the lesions in folia 10 or 11, because these folia can fall off in coronal sections, making it difficult to ascertain for sure whether they were part of the lesion. However, we do not think they were lesioned in our monkeys. It is also exceedingly difficult to assign numbers to the folia of the ventral paraflocculus when some of the middle folia are missing, as they were in many of our cases.
FIG. 2.
Schematic drawing summarizing all the lesions of the floccular complex in our 5 monkeys and in the 2 monkeys from Lisberger et al. (1984). Each square represents one folium on one side of the brain in one monkey. Black, gray, and white filling indicate folia that were completely absent, partially present, or intact. Folia 1– 4 comprise the flocculus, and folia 5–9 are part of the ventral paraflocculus. The lesions are grouped according to the extent of the lesions. Two lesions of the flocculus are shown in the group at the left of the figure (Group I), 2 unilateral lesions of ventral paraflocculus are shown in the middle group (Group II), and 3 large lesions including both flocculus and ventral paraflocculus are shown in the group at the right of the figure (Group III).
Two of the seven monkeys (Ca and Gu) had surgical lesions that were largely restricted to the flocculus and removed only small amounts of ventral paraflocculus. Two monkeys (El and Ka) had neurotoxin lesions that were unilateral and incomplete, but restricted mainly to the ventral paraflocculus. Of note, although 3-NP injections were made bilaterally in each of these monkeys, the damage was primarily restricted to one hemisphere. Extensive lesions were made in three monkeys: one from our current series (Da) received a surgical lesion and neurotoxin injections; two from the report of Lisberger et al. (1984) (Cl and Cr) had large bilateral surgical resection comprising the bulk of the floccular complex. All the lesions, including the extensive one from our series and the two from Lisberger et al. (1984), spared folia in the more rostral part of the ventral paraflocculus and the most caudal folium of the flocculus.
Gross motor deficits
No persistent neurological deficit was found in any of the monkeys. A transient ataxia and nystagmus were usually present during the early postoperative period (1st 48 h). At the time of the first eye movement recording, however, neither the ataxia nor the nystagmus remained. Further, we did not observe the downbeat nystagmus that is characteristic of prior lesions in monkeys (Zee et al. 1981). The only exception was monkey Ca, who had a persistent postsaccadic drift. The neurotoxin lesions sometimes caused a horizontal nystagmus immediately after the injection, but only for a period of minutes.
Bilateral ablations of the flocculus
The lesions in monkeys Ca (Fig. 3B) and Gu (not shown) were similar and bilateral. In both animals, the flocculus was almost entirely removed while the ventral paraflocculus was almost entirely intact. Only in monkey Gu was lobule 1 completely spared, bilaterally. No deficit in visually guided or vestibular eye movement could be detected in monkey Ca and Gu in spite of the large lesions of the flocculus. In particular, the averaged smooth eye velocity did not differ between prelesion (solid lines) and postlesion (dashed lines) responses to step-ramp target motions at 10, 20, or 30°/s in either monkey (Fig. 4, A and B). There was no deficit in vertical pursuit for step-ramp target motion, in tracking of vertical or horizontal sinusoidal target motion, in cancellation of the VOR, or in the VOR in the dark for sinusoidal head motion over the frequency range 0.5–8 Hz (data not shown). When tested with pulses of head velocity, the gain of the VOR in the dark increased slightly in monkey Gu (pre, 0.99 ± 0.01; post, 1.1 ± 0.02, mean ± SE) and decreased slightly in monkey Ca (pre, 1.1 ± 0.019; post, 1.05 ± 0.05).
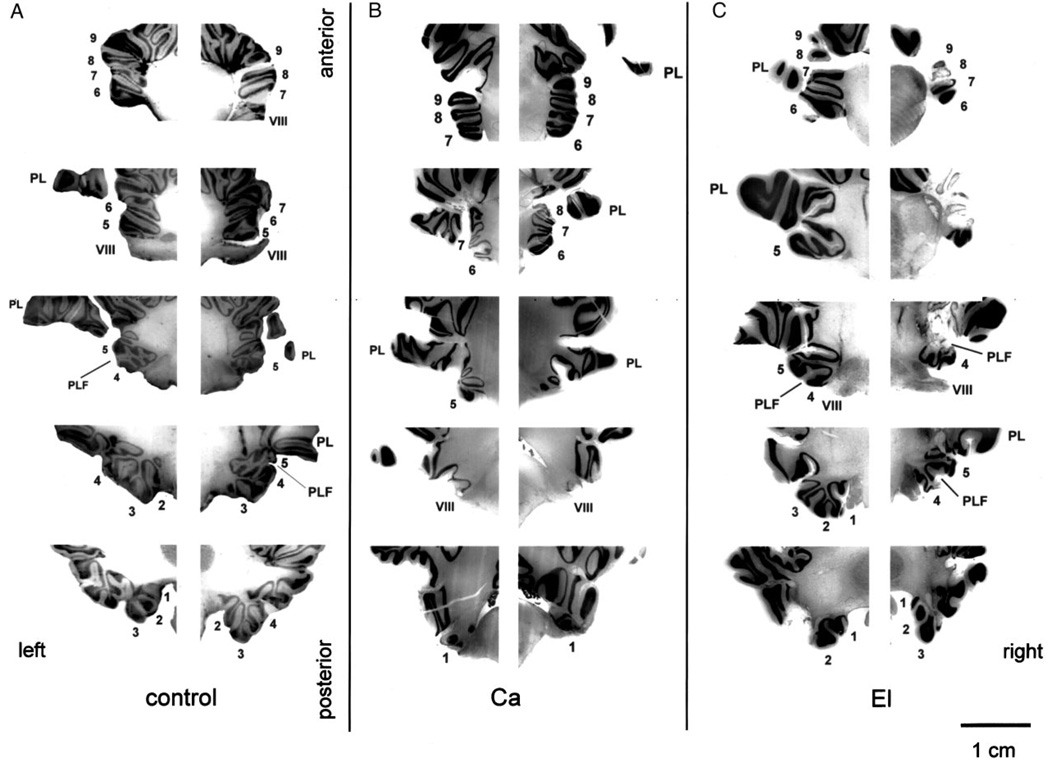
FIG. 3.
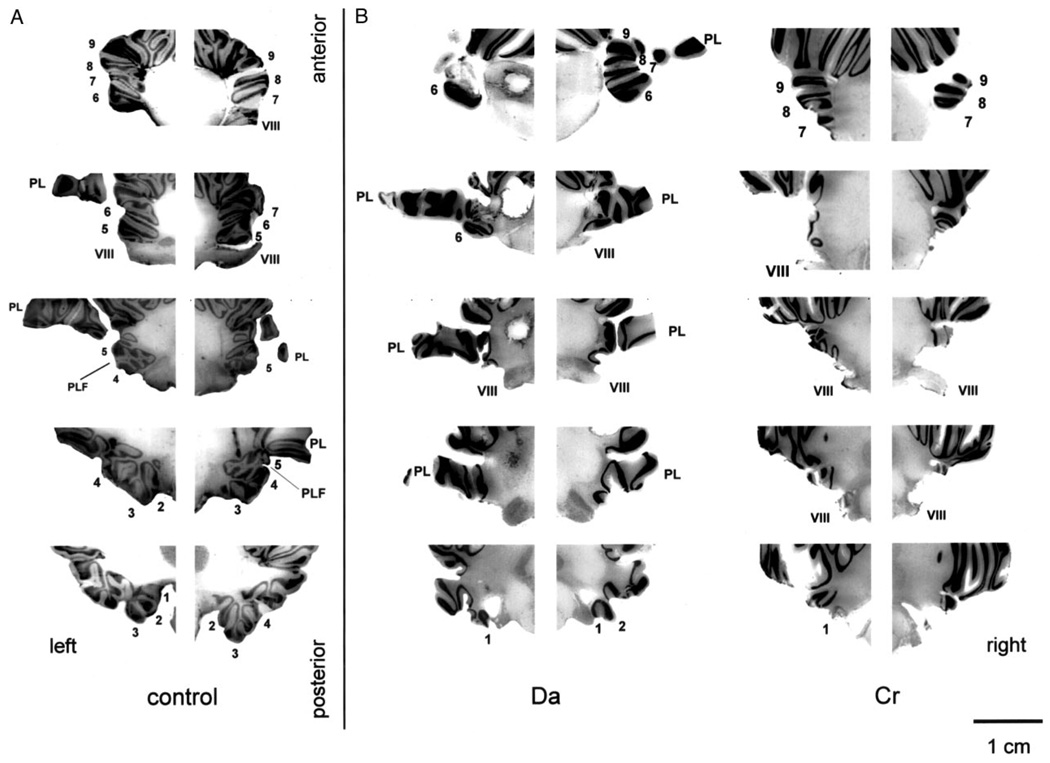
Coronal sections of the right and left floccular-complex in a control monkey (A), a monkey with a bilateral lesion of the flocculus (B), and a monkey with a unilateral lesion of the ventral paraflocculus (C). From top to bottom the sections proceed from rostral to caudal, and each row of sections was taken from the same rostral-caudal level in different monkeys. The numbers alongside each section indicate the folia of the flocculus and ventral paraflocculus that are present. VIII, vestibular nerve; PL, the petrosal lobule; PLF, postero-lateral fissure.
FIG. 4.
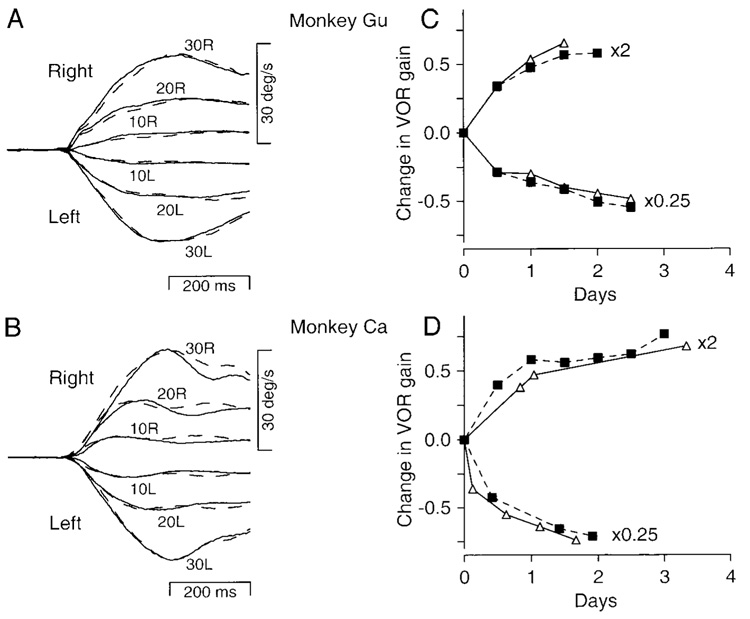
Absence of deficits in smooth pursuit eye movements or motor learning in the vestibuloocular reflex (VOR) of 2 monkeys with bilateral lesions of the flocculus. The top and bottom panels show results for monkeys Gu and Ca, respectively. A and B: averages of smooth eye velocity as a function of time for target motion to the right and left at 10, 20, and 30°/s. Solid and dashed traces show prelesion and postlesion data, respectively. Upward deflections show rightward eye velocity. Numbers next to the traces indicate the ramp target velocity for step-ramp target motions. C and D: gain of the VOR in the dark is plotted as a function of the day of the recording. “×2” and “×0.25” indicate results for spectacles that magnified or miniaturized vision. Open symbols and solid traces show the time course of motor learning before the lesion. Filled symbols and dashed traces show the time course of motor learning after the lesion. Note that the y-axis plots the change in the gain of the VOR relative to the control value before the spectacles were placed on the monkey for each adaptation.
Bilateral ablation of the flocculus also did not alter the VOR adaptation caused by 2–4 days of spectacle adaptation in the home cage. As shown in Fig. 4, C and D, the gain of the VOR followed the same time course and amplitude of changes before (open symbols, solid lines) and after (filled symbols, dashed lines) the ablation, for adaptation with both magnifying (×2) and miniaturizing (×0.25) lenses.
Unilateral lesions of the ventral paraflocculus
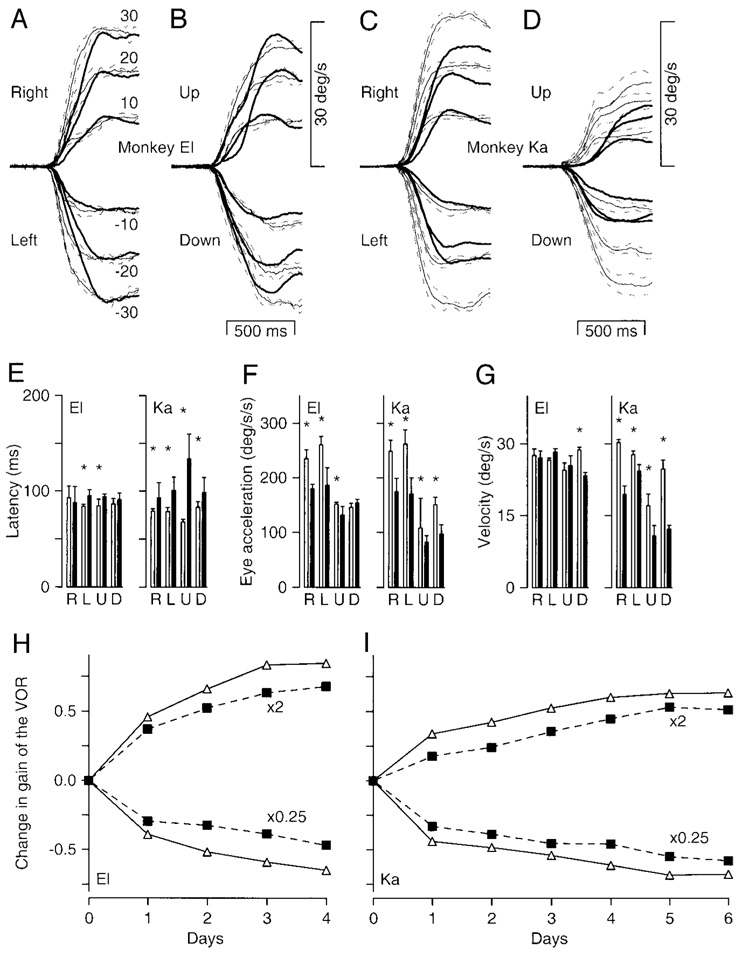
Similar unilateral lesions of the ventral paraflocculus with minor involvement of the flocculus were obtained in both El and Ka. In monkey El the partial floccular lesion affected lobule 3 on the left, and lobule 4 on the right (Fig. 3C). In monkey Ka the flocculus was essentially intact bilaterally, with partial damage to lobule 4 on the right.
In both monkeys, we recorded small deficits in smooth pursuit eye movements and VOR adaptation. For example, Fig. 5, A–D, compares the prelesion (fine traces with SDs) and postlesion (bold traces) time course of averaged eye velocity for step-ramp target motion at 10, 20, and 30°/s. In monkey El, horizontal eye velocity (Fig. 5A) showed deficits in the initiation but not the maintenance of pursuit, while vertical eye velocity (Fig. 5B) showed deficits mainly in the initiation of upward pursuit. In monkey Ka, there were deficits in both the initiation and maintenance of horizontal and vertical pursuit (Fig. 5, C and D). It is noteworthy that the deficits in horizontal pursuit appear to be symmetrical for target motion toward versus away from the side of the lesion in both monkeys. Quantification of these deficits revealed statistically significant increases in latency (Fig. 5E) in both monkeys (except for rightward and downward pursuit in El), statistically significant decreases in eye acceleration at the initiation of pursuit (Fig. 5F) in both monkeys (except for downward pursuit in El), and statistically significant decreases in sustained eye speed (Fig. 5G) in monkey Ka. In these bar graphs, statistically significant differences between prelesion (open bars) and postlesion (filled bars) data are indicated by an asterisk over the pair of bars. In agreement with the deficits in sustained eye velocity, testing with sinusoidal target and head motion revealed postlesion deficits in tracking in monkey Ka, but not in monkey El (data not shown). Deficits were seen across the range of frequencies tested and were similar for pursuit with the head stationary and cancellation of the VOR.
FIG. 5.
Small deficits in smooth pursuit eye movements and motor learning in the VOR of 2 monkeys with unilateral lesions of the ventral paraflocculus. A–D: averages of smooth eye velocity as a function of time for horizontal (A and C) and vertical (B and D) target motion at 10, 20, and 30°/s. The 2 monkeys are shown as the left and right pairs of traces. Fine traces with SDs show prelesion data, and bold traces show postlesion data. Upward deflections show rightward eye velocity. Numbers next to the traces in A indicate the ramp target speed for step-ramp target motions. E–G: bar graphs quantifying parameters of smooth pursuit by showing the mean and SD of latency (E), initial eye acceleration (F), and steady-state eye velocity (G) for pursuit of step-ramp target motion at 30°/s. The 2 graphs in each panel show data for the 2 monkeys. Open and filled bars show data from before and after the lesion. Asterisks indicate statistically significant (P < 0.05) effects of the lesion. H and I: gain of the VOR in the dark is plotted as a function of the day of the recording. “×2” and “×0.25” indicate results for spectacles that magnified or miniaturized vision. Open symbols and solid traces show the time course of motor learning before the lesion. Filled symbols and dashed traces show the time course of motor learning after the lesion. Note that the y-axis plots the change in the gain of the VOR relative to the control value before the spectacles were placed on the monkey for each adaptation.
Small deficits in VOR adaptation were also uncovered in monkeys El and Ka. As illustrated in Fig. 5, H and I, the time course of learning was similar before (open symbols, solid lines) and after (filled symbols, dashed lines) the lesion, but the amplitude of the changes was consistently slightly smaller after the lesion. The deficit was similar for adaptations that increased (×2) and decreased (×0.25) the gain of the VOR. Two facts suggest that these small deficits are real. 1) Because of the small standard errors of the estimates of gain (<0.06 in all cases except 2), the effects of the lesions were statistically significant for all of the individual test days (Mann-Whitney U test, P < 0.05). 2) When tested with sinusoidal head velocities, the deficit in VOR adaptation was similar across testing frequencies (data not shown). Further, the absence of similar deficits using the same adaptation protocol in the monkeys with surgical lesions of the flocculus argues that the small deficit probably does not reflect savings due to repeated adaptations. Finally, recordings of the normal VOR before adaptation with spectacles indicate that the deficit probably was not due to lesions in the brain stem: the control gain of the VOR increased slightly in monkey El (pre, 0.94 ± 0.02; post, 1.05 ± 0.05) and did not change in monkey Ka (pre, 1.1 ± 0.05; post, 1.1 ± 0.02).
Large bilateral lesions including the ventral paraflocculus and flocculus
Examples of the largest lesions of the floccular complex appear in Fig. 6B. Monkey Da underwent a surgical lesion of the flocculus followed by a neurotoxin lesion of the ventral paraflocculus. The combined lesion extended through the flocculus to the ventral paraflocculus on both sides. Only lobule 1 in the right flocculus and lobules 6–9 in the right ventral paraflocculus were spared. A large hole in the white matter of the cerebellum (top 3 sections, left side) is also apparent, probably due to injection of the neurotoxin at one inappropriate site. Monkey Cr is one of the two monkeys published by Lisberger et al. (1984). The surgical lesion in this subject was similar to the combined surgical/neurotoxin lesion in monkey Da, except that the white matter in Cr is intact. In Cr, lobules 7–9 were spared in the ventral paraflocculus on both sides, as well as lobule 1 in the left flocculus. The other monkey from Lisberger et al. (1984) (Cl, Fig. 2) had a lesion that spared lobule 1 in the left flocculus, lobules 8 and 9 in the left ventral paraflocculus, and lobule 1 partially in the right flocculus. Both Cr and Cl also had large lesions of the dorsal paraflocculus that were necessary to expose the ventral paraflocculus for surgical ablation, but monkey Da did not. We characterize these three cases as bilateral lesions involving large portions of the flocculus and ventral paraflocculus. Of these, we present behavioral data only for the new monkey added by our series, Da.
FIG. 6.
Coronal sections of the right and left floccular-complex in a control monkey (A) and 2 monkeys with large bilateral lesions of the flocculus and ventral paraflocculus (B). Monkey Da (middle column) is one of our monkeys, and monkey Cr (right column) is a monkey from Lisberger et al. (1984). From top to bottom the sections proceed from rostral to caudal; each row of sections was taken from the same rostral-caudal level. The numbers alongside each section indicate the folia of the flocculus and ventral paraflocculus that are present. VIII, vestibular nerve; PL, the petrosal lobule; PLF, postero-lateral fissure.
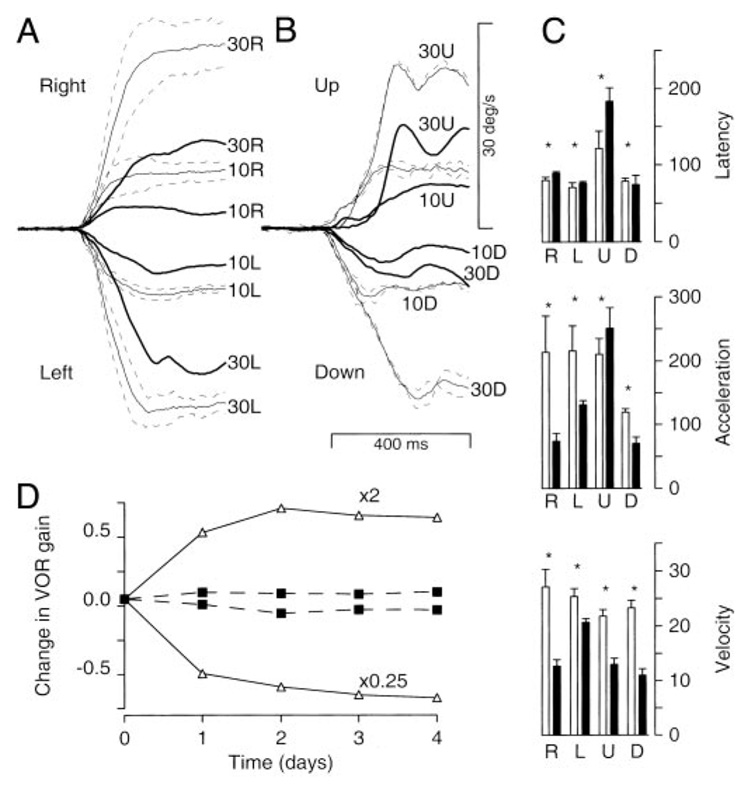
Monkey Da showed a severe deficit in smooth pursuit of step-ramp target motions. The averages of eye velocity in Fig. 7, A and B, reveal deficits in the initiation of pursuit and in steady-state eye velocity for both directions of horizontal (Fig. 7A) and vertical (Fig. 7B) pursuit. Quantitative analysis of the responses for target motion at 30°/s (Fig. 7C) revealed statistically significant increases in latency (top bar graph), decreases in initial eye acceleration at the initiation of pursuit (middle bar graph), and decreases in steady-state eye velocity (bottom bar graph) in all four directions. Although the increases in latency were mostly small, the other two parameters were reduced by as much as 50%. The only exception was upward target motion, when the very long latency resulted in an apparent increase in eye acceleration. We think this is an artifact due to an acceleration so slow that the onset of pursuit was misestimated by the algorithm we used (see METHODS). Indeed, inspection of the traces reveals a somewhat increased latency and very weak initial eye acceleration for upward pursuit after the lesion, followed by a brisk acceleration (Fig. 7B). Analysis of the responses to sinusoidal target motions revealed deficits in both smooth pursuit and cancellation of the VOR (data not shown).
FIG. 7.
Large deficits in smooth pursuit eye movements and motor learning in the VOR in a monkey with large bilateral lesions of the flocculus and ventral paraflocculus. A and B: averages of smooth eye velocity as a function of time for horizontal (A) and vertical (B) target motion at 10 and 30°/s. Fine traces with SDs show prelesion data, and bold traces show postlesion data. Upward deflections show rightward eye velocity. Numbers next to the traces indicate the ramp target speed for step-ramp target motions. C: bar graphs quantifying parameters of smooth pursuit by showing the mean and SD of latency (top), initial eye acceleration (middle), and steady-state eye velocity (bottom) for pursuit of step-ramp target motion at 30°/s. Open and filled bars show data from before and after the lesion. Asterisks indicate statistically significant (P < 0.05) effects of the lesion. D: gain of the VOR in the dark is plotted as a function of the day of the recording. “×2” and “×0.25” indicate results for spectacles that magnified or miniaturized vision. Open symbols and solid traces show the time course of motor learning before the lesion. Filled symbols and dashed traces show the time course of motor learning after the lesion. Note that the y-axis plots the change in the gain of the VOR relative to the control value before the spectacles were placed on the monkey for each adaptation.
VOR adaptation was abolished after the combined surgical and chemical lesion in monkey Da. As shown in Fig. 7D, normal VOR adaptation was observed after the monkey wore both magnifying (×2) and miniaturizing (×0.25) goggles before the lesion (open symbols, solid lines). After the lesion, however, even 4 days of adaptation left the VOR unchanged (filled symbols, dashed lines). In this subject, the surgical lesion caused a slight decrease in the control VOR gain measured in the dark (pre, 1.02 ± 0.03; post, 0.91 ± 0.02), but over the next several months of chemical lesions, the gain of the VOR declined gradually to 0.64. In the two monkeys studied by Lisberger et al. (1984), the deficit in VOR adaptation was complete for Cl and incomplete but severe for Cr (Lisberger et al. 1984). The control gain of the VOR in the dark decreased slightly for Cl, while for Cr the gain was slightly elevated and recovered eventually to normal values.
We also recorded deficits in pursuit and VOR adaptation in monkey Da after surgical lesion but before neurotoxin lesion. Based on the view through the microscope during the surgery, we think the surgical lesion included the flocculus and the most caudal folia of the ventral paraflocculus. Because of the subsequent neurotoxin lesions, however, the histological sections do not reveal the exact surgical lesion, and, for that reason, the data are not included in our graphs. Still, it is worth noting that deficits were present in both pursuit and VOR adaptation and were about the same size or slightly greater than those in the monkeys with the unilateral lesions of the ventral paraflocculus.
Quantitative comparison of lesion size and behavioral deficit in VOR adaptation and pursuit
Table 1 summarizes the deficits in pursuit and VOR adaptation and the size of the lesion for the five monkeys whose lesions are reported in this paper. There are six rows in Table 1 because we have included the behavioral deficits after both the surgical lesion and the total lesion in monkey Da, even though we were able to reconstruct only the total lesion. For both behaviors, we computed the percentage deficit (PD) as
where B is the measure of the behavior. For the VOR, we used the data for the first 4 days of adaptation, since all adaptations (except 1) were at least 4 days long, and B was the change in VOR gain from the control value for each day. We computed PD for each day, and Table 1 reports the mean value for adaptations that increased and decreased the gain of the VOR. For pursuit, we used the data from target motion at 10 and 30°/s, since these were used in all our experiments, and we averaged two estimates of B. One estimate was taken from the initiation of pursuit as the mean eye acceleration over the interval from 120 to 200 ms after the onset of target motion. The other estimate was taken from the maintenance of pursuit as the eye velocity in the interval from 300 to 500 ms after the onset of target motion. We realize that this approach to estimating B obscures the fact that some monkeys showed greater deficits in one phase of pursuit than the other, but we did not have enough cases to test whether the different kinds of deficits resulted from lesions of different regions of the floccular complex. For the analysis of pursuit, we used only the responses to horizontal target motion so that we could compare directly with the VOR evoked by horizontal head rotation. The size of the lesion was estimated by counting the number of folia that were totally removed, or severely compromised, in the different divisions of the floccular complex.
TABLE 1.
Quantitative summary of behavioral deficits and number of folia lesioned in each of our monkeys
| Behavioral Deficits | Lesion Sizes | ||||
|---|---|---|---|---|---|
| Monkey | VOR learning, % | Pursuit, % | Flocculus | Ventral paraflocculus | Total |
| El | −26 | −11 | 2 | 5 | 7 |
| Ka | −29 | −20 | 1 | 5 | 6 |
| Ca | 1 | 3 | 8 | 2 | 10 |
| Gu | 5 | −8 | 6 | 2 | 8 |
| Da total | −90 | −54 | 7 | 6 | 13 |
| Da surgical | −63 | −20.4 | N.A. | N.A. | N.A. |
Negative values of behavioral deficits indicate reduced performance. Lesion sizes are quantified as the number of folia that had extensive damage. Flocculus was defined as folia 1–4 and ventral paraflocculus as folia 5–10. VOR, vestibuloocular reflex. N.A. indicates that the lesion size was not known for the surgical lesion in monkey Da, since we also made chemical lesions in this monkey.
Correlation analysis of the data in Table 1 revealed a strong correlation between the size of the deficits in VOR adaptation and pursuit (Table 2). Regression analysis, on the assumption of much greater variance in the pursuit behavior than in the gain of the VOR, revealed that the deficit in the VOR was 1.77 times greater than that in pursuit. Substitution of a 100% deficit in VOR adaptation into the regression equation implied that a complete deficit in VOR adaptation would be associated with a 50% deficit in pursuit. Correlation of the size of each deficit with the size of the lesion revealed no correlation with the number of folia damaged in the flocculus (folia 1–4), a strong correlation with the number of folia damaged in the ventral paraflocculus (folia 5–9), and an intermediate but statistically nonsignificant correlation with the total number of folia damaged in total floccular complex (Table 2). We included the behavioral data after the surgical lesion in monkey Da in the correlation analysis for the behaviors, but not in the analysis of the relationship between the deficits in behavior and the size of the lesion.
TABLE 2.
Quantitative correlation of behavioral deficits and lesion size
| Measurement | VOR Learning Deficit | Pursuit Deficit |
|---|---|---|
| VOR learning deficit | 0.91 | |
| Pursuit deficit | 0.91 | |
| Lesion size: flocculus | 0.01 | 0.04 |
| Lesion size: ventral paraflocculus | 0.87 | 0.79 |
| Lesion size total | 0.60 | 0.58 |
Each value gives the correlation coefficient based on the measurements summarized in Table 1. VOR, vestibuloocular reflex.
DISCUSSION
Previous lesion experiments on the floccular complex have shown that large, nearly complete lesions cause deficits in smooth pursuit eye movements (Zee et al. 1981) and VOR adaptation (Lisberger et al. 1984). The current results provide the opportunity to examine the results of lesions on a finer scale, and to evaluate how tightly the deficits in the two behaviors were linked. In addition to confirming the large deficits caused by large lesions of the floccular complex, we have shown the following. 1) Deficits in pursuit eye movements and in VOR adaptation are tightly linked. 2) Lesions of the flocculus alone do not cause deficits in pursuit, the control VOR, or VOR adaptation. 3) Unilateral lesions of the ventral paraflocculus have a small effect on both pursuit eye movements and motor learning in the VOR. Taken together with the prior lesion experiments on monkeys, our data imply that the ventral paraflocculus is sufficient to support both VOR adaptation and pursuit even in the absence of the flocculus. This suggests that Purkinje cells in the ventral paraflocculus participate in VOR adaptation, and that it is valid to include their response properties in models of VOR adaptation (Lisberger 1994).
Lesion studies are difficult to interpret, and ours is no exception. One problem is that functions once driven by neurons in a lesioned area might be taken over by neighboring areas. In our experiments, we cannot rule out the possibility that a transfer of function occurred by the time we performed eye movement tests. As we discuss again later in this section, we therefore cannot conclude that the flocculus has no role in either behavior. Indeed, we assume that the rapid adaptation of the remaining system is responsible for our failure to create spontaneous nystagmus, even after unilateral lesions. A second problem is that our procedures for creating chemical lesions may have resulted in undesired lesions outside the floccular complex. In prior lesion studies (Lisberger et al. 1984; Zee et al. 1981), the surgical approach removed most of the dorsal paraflocculus in addition to the floccular complex. Although our new surgical lesions spared the dorsal paraflocculus, the one monkey (Da) with a large lesion of the flocculus and ventral paraflocculus also had unintended damage to the cerebellar white matter. This lesion may have disrupted the outputs of other areas of the cerebellar cortex that participate in one or both behaviors we tested, so we cannot be certain that the effects we measured are due to the lesions of the floccular complex only. Our conclusions must be viewed with this potential issue in mind.
Anatomical versus functional differentiation of flocculus and ventral paraflocculus
Recent anatomical experiments have emphasized a number of anatomical differences between the flocculus and ventral paraflocculus, as well as their different embryological origin (Gerrits and Voogd 1985; Nagao et al. 1997a,b; Osanai et al. 1999). Although the floccular complex shows an anatomical subdivision separating lobes 1–4 from lobes 5–11, the data reported here indicate that the anatomical subdivision does not correspond to a functional subdivision of smooth pursuit and VOR adaptation. The ventral paraflocculus is an evolutionarily newer structure, but seems to be able to support normal VOR adaptation, a behavior exhibited by animals spanning a wide variety of evolutionary ages. This finding may suggest that VOR adaptation is so critical for gaze stability that the neurons underlying it are widely distributed across evolutionarily distinct regions.
Our experiment was motivated by Nagao’s (1992) suggestion that the anatomical differences between the flocculus and ventral paraflocculus might have a functional correlate. If his suggestion were correct, then lesions of the flocculus should have impaired VOR adaptation without affecting smooth pursuit eye movements, and lesions of the ventral paraflocculus should have impaired smooth pursuit eye movements without affecting VOR adaptation. Contrary to this prediction, we observed a strong correlation between the sizes of the deficits in VOR adaptation and pursuit, even when lesions were restricted to one sub-region of the floccular complex.
We do not think Nagao’s hypothesis can be rescued on the basis that complete VOR adaptation could be supported by the small amounts of lobules 1 and 2 that remained in our flocculus lesions. The same lobules remained in the two monkeys reported by Lisberger et al. (1984) and in monkey Da, all of whom exhibited dramatic deficits in VOR adaptation. Thus the ventral paraflocculus must play an important role in both VOR adaptation and pursuit. We also do not think it is possible to rescue Nagao’s (1992) hypothesis by the prediction of our data that 50% of pursuit would remain when VOR adaptation was abolished by large lesions of the floccular complex. There are nonfloccular pathways for pursuit in the cerebellum (Fuchs et al. 1994; Westheimer and Blair 1974), so that only a partial deficit in pursuit would be expected even with complete removal of the floccular complex. Thus we argue that our data reject Nagao’s (1992) suggestion: the ventral paraflocculus appears to play equally important roles in pursuit and VOR adaptation. However, it remains possible that lobules 1–4 participate only in VOR adaptation, if 1) their function can be quickly taken over by the ventral paraflocculus when the flocculus is ablated and 2) lobule 1 alone does not contain enough tissue to support VOR adaptation alone. Still, the poor correlation between the size of the behavioral deficit in VOR adaptation and the amount of tissue removed from the flocculus argues against this scenario.
Relevant parts of the floccular complex for VOR adaptation and pursuit
Our data suggest that much of the floccular complex participates in pursuit and motor learning in the VOR, and that larger lesions cause bigger deficits. We suggest that lobules 5–8 are the most important areas of the floccular complex for pursuit eye movements and VOR adaptation, because we observed a strong correlation of the behavior deficits with the size of the lesion in the ventral paraflocculus and no significant correlation with the size of the lesion in the flocculus or the entire floccular complex. Unilateral lesions of the lobules 5–8 were sufficient to cause small deficits in both behaviors, whereas sparing of lobules 1 and 2 or 9 and 10 in the monkeys of Lisberger et al. (1984) and in monkey Da were insufficient to rescue normal behavior.
Even though we found no correlation of behavioral deficit with the size of the lesion in the flocculus, our data do not resolve the question of whether lobules 1–4 of the floccular complex are involved in pursuit or VOR adaptation. First, the results of our lesion study capture only the performance of the system after several days or weeks of recovery from the lesions. The flocculus may participate in the behaviors when it is present, and plasticity may restore behavioral performance quickly after the flocculus is removed. Second, the correlation analysis, because it only counts lesioned lobules, does not take into account that there is much more cerebellar tissue in lobules 5–9 of the floccular complex than in lobules 1–4. Finally, we note that the largest behavioral deficits appeared in cases that lesioned both the flocculus and the paraflocculus [monkey Da, and the 2 monkeys in Lisberger et al. (1984)], indicating that the flocculus may participate in one or both functions. This would be compatible with the finding that horizontal gaze velocity Purkinje cells (HGVP) were recorded in lobules 3–8 of the floccular complex by Lisberger et al. (1994a). We had hoped to determine the role of the flocculus alone by making complete bilateral lesions of the ventral paraflocculus with the neurotoxin, but we were not successful.
Comparison with results of lesions in other species
The point of our study was to evaluate Nagao’s hypothesis about different functions of the flocculus and ventral paraflocculus in monkeys. The evidence in favor of that hypothesis was generated in monkeys (Nagao 1992), and the conclusions that would be undermined if the hypothesis were true also came from research on monkeys (Lisberger 1994). Thus our conclusions are based on data from a single species and can be applied to that species. Because of the differences in the relative size of the flocculus and ventral paraflocculus across species, it is difficult to make a fair comparison. In the rabbit, for example, the flocculus and a single lobule of ventral paraflocculus (Gerrits and Voogd 1989; Yamamoto and Shimoyama 1977) appear to have anatomical connections with the inferior olive and vestibular nucleus that suggest homology to the floccular complex of monkeys. Lesion studies have not attempted to discriminate the different functions of the two parts of the floccular complex in rabbits, but it seems likely that both structures contribute to VOR adaptation and visual tracking, as we agree is probably the case in monkeys. Given the example provided by our monkey data, it also seems likely that partial lesions of the floccular complex in the rabbit would cause linked deficits in visual tracking and VOR adaptation.
Differences in the exact paradigms used to analyze the effects of lesions may have a greater impact on the results. One difference is that many studies have been done using the optokinetic response to evaluate visual tracking rather than pursuit, an appropriate decision in nonprimate species that lack good pursuit tracking. Available evidence in the monkey suggests that pursuit and the optokinetic response provide equivalent assessments of deficits in visual tracking. It seems likely that the floccular complex is far enough downstream in the visual tracking circuits so that its function is independent of the exact stimulus used: this is supported by recordings from floccular Purkinje cells during pursuit (Stone and Lisberger 1990), ocular following (Shidara and Kawano 1993), and optokinetic nystagmus (Büttner and Waespe 1984). Further, lesions of the floccular complex in monkeys cause a deficit in the rapid component of optokinetic nystagmus (Waespe et al. 1983) that parallels the deficit in pursuit.
Another difference is the method used for VOR adaptation. It seems unlikely that the use of passive versus active head turns for adaptation is important, since large floccular ablations caused similar effects when adaptation was done with passive head turns (Lisberger et al. 1984) and with active head turns (monkey Da in present study). However, there is now evidence that short-term and long-term VOR adaptation may employ somewhat different neural mechanisms, since expression of a protein kinase C inhibitor in Purkinje cells prevents short-term adaptation of the VOR (de Zeeuw et al. 1998) while preserving some long-term adaptation (C. I. de Zeeuw, personal communication). Since we made our first measurements of adaptation 24 h after the monkeys first donned the spectacles, our data are relevant only to long-term adaptation, which is clearly affected by ablation of the floccular complex. It is possible that short-term adaptation was abolished by lesions restricted to the flocculus, but that this deficit escaped our analysis.
Finally, we note the interesting observation that large unilateral ablations of the ventral paraflocculus caused some deficit in both pursuit and VOR adaptation, and the fact that the pursuit deficits were present for both ipsi-lesional and contra-lesional pursuit. Unilateral lesions are also effective in rabbits (Ito et al. 1982). The existence of deficits with unilateral lesions agrees with clinical observations on patients with tumors that invade the floccular complex (Reutern and Dichgans 1977; Waespe 1992; Yamazaki and Zee 1979). In humans, however, the lesions cause deficits in ipsi-lesional smooth pursuit and optokinetic responses and in cancellation of the VOR during contra-lesional head motion (Waespe 1992). Human subjects also showed gaze-evoked spontaneous (Waespe 1992) and rebound nystagmus (Yamazaki and Zee 1979) that did not appear strongly in our monkeys. Perhaps the parts of the floccular complex spared by our lesions mediate compensation that ameliorated these deficits before we could quantify them.
Acknowledgments
We thank D. Zee for allowing us to reanalyze and include the histological material from an earlier collaboration and K. MacLeod for expert surgical and animal health support that made the surgical ablations of the flocculus possible. S. G. Lisberger is an investigator of the Howard Hughes Medical Institute (HHMI).
H. Rambold was supported by a fellowship from the Deutsche Forschungs-gemeinschaft. This research was supported by the HHMI and by National Eye Institute Grant EY-03878.
REFERENCES
- Büttner U, Waespe W. Purkinje cell activity in the primate flocculus during optokinetic stimulation, smooth pursuit eye movements, and VOR-suppression. Exp Brain Res. 1984;55:97–104. doi: 10.1007/BF00240502. [DOI] [PubMed] [Google Scholar]
- Carl JR, Gellman RS. Human smooth pursuit: stimulus dependent responses. J Neurophysiol. 1987;57:1446–1463. doi: 10.1152/jn.1987.57.5.1446. [DOI] [PubMed] [Google Scholar]
- Chyi T, Chang C. Temporal evolution of 3-nitropropionic acid-induced neurodegeneration in the rat brain by T2-weighted, diffusion-weighted, and perfusion magnetic resonance imaging. Neuroscience. 1999;92:1035–1041. doi: 10.1016/s0306-4522(99)00076-7. [DOI] [PubMed] [Google Scholar]
- de Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kimm J. Unit activity in the vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol. 1975;38:1140–1161. doi: 10.1152/jn.1975.38.5.1140. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol. 1994;72:2714–2728. doi: 10.1152/jn.1994.72.6.2714. [DOI] [PubMed] [Google Scholar]
- Gerrits NM, Voogd J. The topographical organization of climbing and mossy fiber afferents in the flocculus and ventral paraflocculus in rabbit, cat and monkey. Exp Brain Res Suppl. 1989;17:26–29. [Google Scholar]
- Gonshor A, Melvill Jones G. Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol (Lond) 1976;256:381–414. doi: 10.1113/jphysiol.1976.sp011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Jastreboff PJ, Miyashita Y. Specific effects of unilateral lesion in the flocculus upon eye movements in albino rabbit. Exp Brain Res. 1982;45:233–242. doi: 10.1007/BF00235783. [DOI] [PubMed] [Google Scholar]
- Ito M, Nisimaru N, Yamamoto M. Specific pattern of neuronal connections involved in the control of rabbit’s vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol (Lond) 1977;265:833–854. doi: 10.1113/jphysiol.1977.sp011747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Keller EL. Gain of the vestibulo-ocular-reflex in the monkey at high rotational frequencies. Vision Res. 1978;18:311–315. doi: 10.1016/0042-6989(78)90165-7. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, van Alphen AM, van de Burg J, Grosveld F, Galjart N, de Zeeuw CI. Gain adaptation and phase dynamics of compensatory eye movements in mice. Genes Function. 1997;1:175–190. doi: 10.1046/j.1365-4624.1997.00018.x. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Neural basis for motor learning in the vestibuloocular reflex of primates. III. Computational and behavioral analysis of the sites of learning. J Neurophysiol. 1994;72:974–998. doi: 10.1152/jn.1994.72.2.974. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF. Role of the primate flocculus during rapid behavioral adaptation of the vestibulo ocular reflex. I. Purkinje cell activity during visually guided horizontal smooth pursuit eye movements and passive head rotations. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Miles FA, Zee DS. Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J Neurophysiol. 1984;52:1140–1153. doi: 10.1152/jn.1984.52.6.1140. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA. Vestibular signals carried by pathways subserving plasticity of the vestibulo-ocular reflex in monkeys. J Neurosci. 1986;6:346–354. doi: 10.1523/JNEUROSCI.06-02-00346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Bronté-Stewart HM, Stone LS. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the response of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol. 1994a;72:954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the response of brainstem neurons. J Neurophysiol. 1994b;72:928–953. doi: 10.1152/jn.1994.72.2.928. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott JG, Beeton P, Polk J. Effects of the cerebellar inactivation by lidocaine microdialysis on the vestibuloocular reflex in goldfish. J Neurophysiol. 1998;79:1286–1294. doi: 10.1152/jn.1998.79.3.1286. [DOI] [PubMed] [Google Scholar]
- Michnovitz JJ, Bennett MVL. Effects of rapid cerebellectomy on adaptive gain control of the vestibulo-ocular reflex in alert goldfish. Exp Brain Res. 1987;66:287–294. doi: 10.1007/BF00243305. [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB. Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol. 1980;43:1406–1425. doi: 10.1152/jn.1980.43.5.1406. [DOI] [PubMed] [Google Scholar]
- Miles FA, Fuller JH. Adaptive plasticity in the vestibulo-ocular responses of the rhesus monkey. Brain Res. 1974;80:512–516. doi: 10.1016/0006-8993(74)91035-x. [DOI] [PubMed] [Google Scholar]
- Miles FA, Fuller JH, Braitman DJ, Dow BM. Long-term adaptive changes in the primate vestibuloocular reflex. III. Electrophysiological observation in flocculus of normal monkeys. J Neurophysiol. 1980;43:1473–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Nagao S. Effect of vestibulo-cerebellar lesion upon dynamic characteristics and adaptation of the vestibuloocular and optokinetic responses in pigmented rabbits. Exp Brain Res. 1983;53:36–46. doi: 10.1007/BF00239396. [DOI] [PubMed] [Google Scholar]
- Nagao S. Different roles of flocculus and ventral paraflocculus for oculomotor control in primate. Neuroreport. 1992;3:13–16. doi: 10.1097/00001756-199201000-00003. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitamura T, Nakamura H, Hiramatsu T, Yamada J. Differences of the primate flocculus and ventral paraflocculus in the mossy fiber and climbing fiber input organization. J Comp Neurol. 1997a;382:480–498. doi: 10.1002/(sici)1096-9861(19970616)382:4<480::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitamura T, Nakamura H, Hiramatsu T, Yamada J. Location of efferent terminals of the flocculus and ventral paraflocculus revealed by anterograde axonal transport methods. Neurosci Res. 1997b;27:257–269. doi: 10.1016/s0168-0102(97)01160-7. [DOI] [PubMed] [Google Scholar]
- Olsen C, Rustad A, Fonnum F, Paulsen RE, Hassel B. 3-Nitropropinoic acid: an astrocyte-sparing neurotoxin in vitro. Brain Res. 1999;850:144–153. doi: 10.1016/s0006-8993(99)02115-0. [DOI] [PubMed] [Google Scholar]
- Osanai R, Nagao S, Kitamura T, Kawabata I, Yamada J. Differences in mossy and climbing fiber afferent sources between flocculus and ventral and dorsal paraflocculus in the rat. Exp Brain Res. 1999;124:248–264. doi: 10.1007/s002210050620. [DOI] [PubMed] [Google Scholar]
- Pang Z, Geddes JW. Mechanisms of cell death induced by the mitochondrial toxin 3-nitropopionic acid: acute excitotoxic necrosis and delayed apoptosis. J Neurosci. 1997;17:3064–3073. doi: 10.1523/JNEUROSCI.17-09-03064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth pursuit tracking eye movements. J Physiol (Lond) 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutern GM, Dichgans J. Oculomotor disturbances in pontine angle tumors. Contralateral diminution of optokinetic nystagmus as early sign of floccular lesions. Arch Psychiatr Nervenkr. 1977;223:117–130. doi: 10.1007/BF00345951. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976;39:954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- Shidara M, Kawano K. Role of Purkinje cells in the ventral paraflocculus in short-latency ocular following responses. Exp Brain Res. 1993;93:185–195. doi: 10.1007/BF00228385. [DOI] [PubMed] [Google Scholar]
- Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- Waespe W. Deficits of smooth-pursuit eye movements in two patients with a lesion in the (para-) floccular or dorsolateral pontine region. Neuro-ophthalmology. 1992;12:91–96. [Google Scholar]
- Waespe W, Cohen B, Raphan T. Role of the flocculus and paraflocculus in optokinetic nystagmus and visual-vestibular interactions: effects of lesions. Exp Brain Res. 1983;50:9–33. doi: 10.1007/BF00238229. [DOI] [PubMed] [Google Scholar]
- Westheimer G, Blair SM. Functional organization of primate oculomotor system revealed by cerebellectomy. Exp Brain Res. 1974;21:463–472. doi: 10.1007/BF00237165. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Shimoyama I. Differential localization of rabbit’s flocculus Purkinje cells projecting to the medial and superior vestibular nuclei, investigated by means of the horseradish peroxidase retrograde axonal transport. Neurosci Lett. 1977;5:279–283. doi: 10.1016/0304-3940(77)90079-9. [DOI] [PubMed] [Google Scholar]
- Yamazaki A, Zee DS. Rebound nystagmus: EOG analysis of a case with a floccular tumor. Br J Ophthalmol. 1979;63:782–786. doi: 10.1136/bjo.63.11.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee DS, Yamazaki A, Butler PH. Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol. 1981;46:878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]