Abstract
Background and Purpose
Risk for both intracranial aneurysms (IAs) and aortic aneurysms (AAs) is thought to be heritable with mounting evidence for genetic predisposition. The concept of shared risk for these conditions is challenged by differences in age of diagnosis and demographic characteristics. We performed a genomewide linkage analysis in multiplex families with both IA and AA from the Familial Intracranial Aneurysm study.
Methods
Available medical records of subjects who reported IA or abdominal/thoracic AA were reviewed with adjudication as definite/probable, possible, or not a case. To identify genes contributing to the susceptibility for IA and AA, genomewide linkage analysis was performed in the 26 multiplex IA families who had members who also had thoracic or abdominal AA. Individuals (n=91) were defined as affected if they had an IA (definite/probable) or an aortic or thoracic AA (definite/probable).
Results
Maximum logarithm of odds (LOD) scores were found on chromosomes 11 (144 cM; LOD=3.0) and 6 (33 cM; LOD=2.3). In both chromosomal regions, analyses of these same 26 families considering only IA as the disease phenotype produced LOD scores of 1.8 and 1.6, respectively.
Conclusions
Our linkage analysis in these 26 families using the broadest disease phenotype, including IA, abdominal AA, and thoracic AA, supports the concept of shared genetic risk. The chromosome 11 locus appears to confirm earlier independent associations in IA and AA. The chromosome 6 finding is novel. Both warrant further investigation.
Keywords: aortic aneurysms, genetic linkage, genetic susceptibility, intracranial aneurysm, single nucleotide polymorphism
Risk for both intracranial aneurysms (IAs) and aortic aneurysms (AAs) is thought to be heritable with mounting evidence in both for genetic predisposition.1,2 Both conditions share a number of associated risk factors such as smoking and hypertension. Co-occurrence of IAs and AAs within pedigrees is estimated to be 10.5%.3 Differences in age of diagnosis and demographic characteristics between the 2 conditions have led some to assume that such co-occurrence within a single pedigree was simply due to chance. However, familial aggregation studies suggest that shared genetic risk factors may contribute to IA and AA susceptibility.3 To test this hypothesis, we performed linkage analysis in a subset of the multiplex IA families collected as part of the international Familial Intracranial Aneurysm study that segregated both IA and AA aneurysms in extracerebral vessels, including the thoracic and abdominal aorta.
Methods
Subjects
The Familial Intracranial Aneurysm (FIA) study protocol4 and the initial genomewide linkage scan5 have been previously published. Briefly, probands were recruited through 41 experienced sites throughout North America, New Zealand, and Australia. Eligible families included those with either 2 or more siblings with an IA, at least one of whom is living and the other whose genotype could be reconstructed, or 3 or more affected family members with at least one of whom is living and at least one other affected relative whose genotype could be reconstructed. The FIA study was approved by the Institutional Review Boards/ethics committees at all clinical and analytic centers and recruitment sites.
Phenotyping
Intracranial aneurysm status was determined by 2 neurologists who independently reviewed the subjects’ records and determined if the subject met all the inclusion and exclusion criteria. In cases of disagreement, a third neurologist was used to resolve the case diagnosis. Each potential affected family member was ranked as definite, probable, possible, or not a case (Table 1). Questionnaire data regarding demographics, environmental risk factors, and family history of IA were obtained from persons with an IA and their unaffected family members. This included information on aneurysms in other vascular beds.
Table 1.
Phenotype Definitions
| IA | AA | |
|---|---|---|
| Definite |
|
• Medical records document TAA or AAA diagnosed by CT, arteriogram, ultrasound, or visualized during operation or autopsy |
| Probable |
|
• TAA or AAA referenced in medical records with specific details |
| Possible |
|
• TAA or AAA diagnosed on chest or abdominal x-ray only or reported on medical history questionnaire or yearly follow-up without supporting documentation |
| Not case | • There is no supporting information for a possible IA | • There is no supporting information for a possible AA; no mention of aneurysm in medical records or aneurysm located outside abdominal/thoracic region (for example, splenic aneurysm) |
All medical records and a phone screen of the proband and family members reporting an aortic, abdominal, or thoracic aneurysm were examined by 2 neurologists who independently reviewed the records to determine if the subject had an abdominal or thoracic AA and recorded location, size, and rupture status. In cases of disagreement, a third physician was used as a tiebreaker. Each subject was ranked as definite/probable, possible, or not a case (no mention of aneurysm in records or aneurysm located outside abdominal or thoracic region) as outlined in Table 1.
Genotyping
Blood was obtained from all participants for the isolation of DNA. The Center for Inherited Disease Research operated by National Human Genome Research Institute (NHGRI) genotyped 2317 individuals from 394 families using the 6K Illumina array. The error rate, based on paired genotypes from 107 duplicate samples, was 0.0022%. The percentage of missing genotypic data was 0.24%.
Statistical Analysis
Demographic and risk factor data were compared between those with IA and those with AA. Due to the anticipated difference in age at diagnosis for IA and AA, values were age-adjusted. For continuous risk factors, age was treated as a continuous covariate. For categorical risk factors, age at diagnosis was dichotomized as ≤65 versus ≥66 years. Analysis of covariance was used for continuous risk factors and Cochran-Mantel-Haenszel χ2 tests with 2 age strata were used for categorical risk factors.
Detailed quality assessment was performed in genotypic data received from The Center for Inherited Disease Research before initiating analyses. All family relationships were verified6 and pedigree structures were altered as necessary. All markers with fewer than 90% of the individuals genotyped and all samples with fewer than 90% of the markers genotyped were removed from further analyses. Markers violating Hardy-Weinberg equilibrium at P<0.001 or with very low informativeness (minor allele frequency <0.05) were removed from further analysis. Mendelian errors in each family were reviewed and genotypes were removed as needed to eliminate inconsistencies. Final genotypic quality control focused on removing any remaining genotypes that were likely to be erroneous as determined by evidence of recombination with adjacent markers (22). Before performing any linkage analyses, the linkage disequilibrium between single nucleotide polymorphisms was evaluated and in all cases where D’ exceeded 0.70, the single nucleotide polymorphism with the highest minor allele frequency (ie, the most informative marker) was preferentially retained. The final sample consisted of 333 multiplex IA families consisting of 1895 samples.
To evaluate the evidence that common genes affect both IA and AA, multipoint, model-independent linkage analyses were performed using 2 models of disease. The first and broadest disease definition classified as affected only those individuals who met the definite or probable criteria for either IA or AA in abdominal or thoracic locations. It has been suggested that thoracic AAs (TAA) may be more heritable and are more strongly linked to known genetic conditions such as Marfan’s syndrome than abdominal aortic aneurysms (AAA)7; therefore, a second, narrower disease definition that classified as affected only those individuals who met criteria for definite IA and/or those who met criteria for definite or probable thoracic AA was used.
Results
As of September 1, 2007, 2847 individuals from 546 families were in the database. Both definite/probable IA and AA were found in 11 individuals; 19 had definite/probable AA but no history of IA. In addition, 2 subjects had definite/probable AA and a possible IA, 2 subjects had definite/probable IA and possible AA, and 3 had possible AA but no IA. Of the 11 individuals with both conditions, 2 had their AA diagnosed first, 4 their IA diagnosed first, 4 were diagnosed made within the same year, and the age of AA diagnosis was not available for one subject.
Comparison of baseline characteristics of subjects with IA and those with AA are presented in Table 2. As expected, the average age of diagnosis for IA was substantially younger that for AA. Age adjustment had a substantial effect on pack-years and duration of hypertension.
Table 2.
Demographic and Risk Factor Characteristic Comparison of Subjects With Diagnosed IA to Diagnosed TAA/AAA*
| Variable | IA (n = 1119) | TAA/AAA (N = 19) | P Value | Age-Adjusted P Value |
|---|---|---|---|---|
| Race, white | 91.2% | 100% | 0.399 | N/A |
| Sex, male | 23.9% | 52.6% | 0.012 | 0.001 |
| Smoking | ||||
| Current | 50.3% | 36.8% | 0.382 | 0.601 |
| Former | 27.8% | 42.1% | ||
| Never | 21.9% | 21.1% | ||
| Hypertensive | 46.4% | 73.7% | 0.021 | 0.017 |
| Frequent alcohol | 39.3% | 50.0% | 0.648 | 0.557 |
| Diabetes | 7.6% | 15.8% | 0.176 | 0.399 |
| High cholesterol | 34.5% | 88.2% | <.001 | <0.001 |
| Myocardial infarction or atrial fibrillation | 7.5% | 31.6% | 0.002 | 0.007 |
| Age at diagnosis, years | 49.8 (SD, 12.1) | 67.2 (SD, 11.3) | <.001 | N/A |
| Cigarettes/day | 16.7 (SD, 13.6) 16.7† |
18.3 (SD, 9.6) 18.3† |
0.667 | 0.656 |
| Pack-years of cigarettes | 23.9 (SD, 21.8) 24.0† |
33.3 (SD, 15.5) 25.7† |
0.095 | 0.759 |
| Daily alcohol, drinks/day | 0.96 (SD, 2.86) 0.96† |
0.38 (SD, 0.84) 0.76† |
0.010 | 0.768 |
| Duration of hypertension | 5.84 (SD, 11.4) 3.41† |
16.6 (SD, 13.8) 9.23† |
<.001 | <0.001 |
Does not include the 11 individuals with both IA and AA.
Denotes age-adjusted means for continuous variables.
NA indicates not applicable.
A total of 28 families had members with AA as well as IA. However, 2 families were not informative for linkage; therefore, they could not be included in the analysis. Thus, linkage analyses were performed in the 26 multiplex IA families who were informative for linkage and who had also had members with thoracic or abdominal AA. Individuals from these 26 families (n=91) were defined as affected in the analysis if they had an IA (definite or probable), a TAA (definite or probable), or an AAA (definite or probable; Table 3). Of those with IA, 33 of 70 (47.1%) had a history of subarachnoid hemorrhage, whereas 5 of 30 (16.7%) with AA had a history of rupture. None of the 9 individuals with both IA and AA had a history of AA rupture, but 4 of 9 had a history of subarachnoid hemorrhage.
Table 3.
Distribution of Aneurysm Types Within the 26 Analyzed Families
| IA | TAA | AAA | No. of Subjects (n = 91) |
|---|---|---|---|
| + | - | - | 61 |
| + | + | - | 3 |
| + | - | + | 6 |
| + | + | + | 0 |
| - | + | - | 3 |
| - | + | + | 2 |
| - | - | + | 16 |
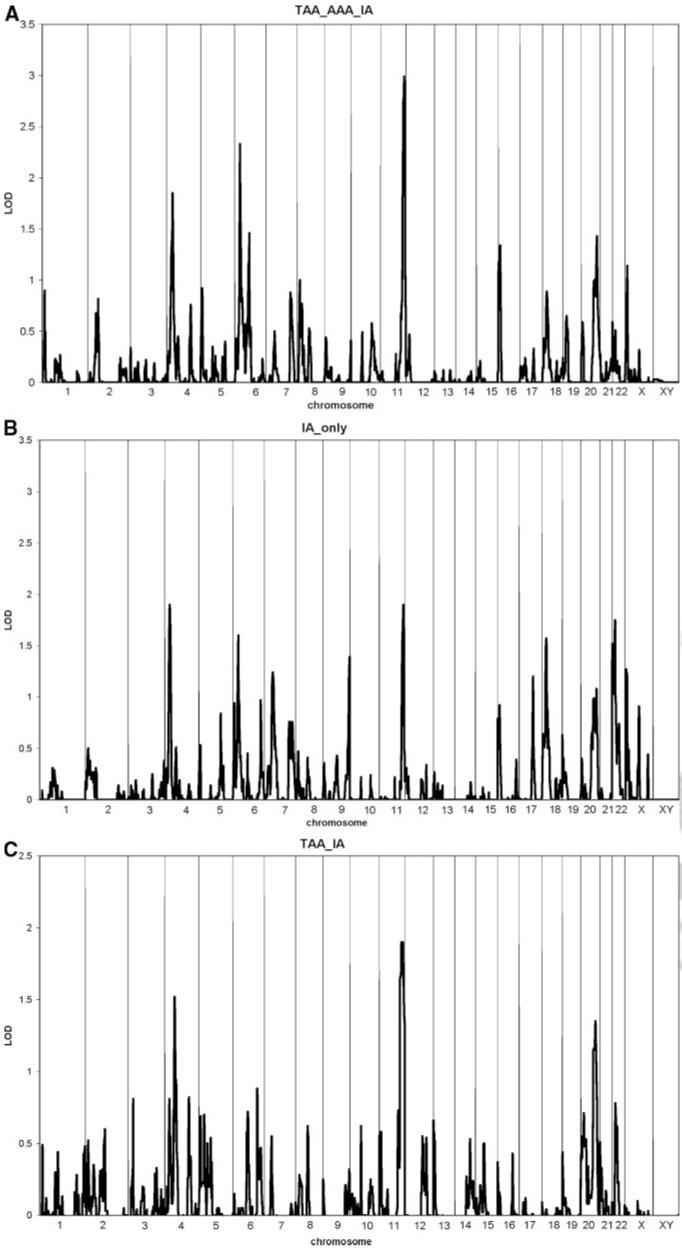
Maximal logarithm of odds (LOD) scores were obtained on chromosome 11 (144 cM; LOD=3.0; Figure A) and chromosome 6 (33 cM; LOD=2.3; Figure B). In both chromosomal regions, analyses of only these same 26 families using only IA as the phenotype produced LOD scores of 1.8 and 1.6, respectively. Restricting the phenotype to thoracic AA or IA resulted in similar, albeit more modest, values (Figure C). Fine mapping of chromosomes 11 and 6 can be found online (Supplemental Figure I, A—B, available online at http://stroke.ahajournals.org).
Figure.
Results of multipoint, model independent linkage analysis. A, Broadest disease definition: IA, AAA, or TAA. B, IA alone in the same families. C, Restricted disease definition: IA or TAA.
Discussion
Although common genetic risk factors for both IA and AA disease have long been speculated,8 the data have been sparse and inconsistent. A pedigree analysis conducted in the Utah Pedigree Database, which links genealogical data to demographic and medical information, including death certificates, found cosegregation of AAs and other aneurysms, although not IAs.9 A very small series of individuals with coronary ectasia were screened with thoracic/abdominal and intracranial MR angiography and 3 were found to have aneurysms.10 A recent treatment-based case-series reported co-occurrence of AA within pedigrees in 10.5% of probands with IA, lending support to the theory of shared genetic risk.3 There are data suggesting that TAA and AAA may have different pathophysiological mechanisms. A recent analysis of risk factor profiles among those with AAA and TAA underscores the importance of considering differences between these disorders.11 However, other data suggest potential shared genetic susceptibility, including a recent analysis of shared loci for TAA, AAA, and IA.12 The fact that there are undoubtedly differences in these 3 disorders does not preclude the potential for shared genetic susceptibility.
In this analysis from the FIA study population, we found a substantial minority of families that reported members with AA. As has been noted in other series, those with AA tend to be older at diagnosis, more likely to be men, and have longstanding hypertension, dyslipidemia, and evidence of heart disease than those with IA. In a genomewide linkage analysis on 26 white families with the co-occurrence or IA and AA, we found 2 peaks on chromosome 11 and chromosome 6 for the broad phenotype of IA or any AA. In the narrow phenotype analysis, we did not see new peaks emerging or evidence that the overall association was driven by thoracic aneurysms. Furthermore, the results of our analysis differ from other recent studies focusing on thoracic disease.13
Our identification of the chromosome 11 locus adds to mounting evidence for a susceptibility gene in this region. This region (11q24-25) has been previously found linked in other studies to aneurysmal disease.14,15 In one study of 2 large pedigrees with IAs, 2 individuals known to harbor AAAs inherited the haplotype linked to IA on chromosome 11. The inclusion of these 2 AAA cases in the linkage analysis increased the LOD score from 3.6 to 4.3.15 The authors interpreted their linkage as confirming a finding from an earlier Japanese sibpair analysis.16 Another study of familial AAs described the FAA1 gene on 11q23–24 between D11S1341 and AFMB031WC9.14 This FAA1 locus has been associated with a more widespread vasculopathic process than other candidates,7 although none of the genes in the region are currently viewed as strong mechanistic candidates for aneurysmal disease.14
Our finding is between 137.3 and 149.3 cM (decode), which is between 128.2 Mbp and 132.5 Mbp. It is in the range of Ozturk et al (125.6 Mbp to 131.4 Mbp). It is approximately 10 Mbp from Vaughan et al (117 Mbp to 120 Mbp); however, key recombinants resulted in a very narrow confidence interval (1.1 cM). This same region was associated with the IA phenotype in a meta-analysis with a probability value of 0.012.17 As outlined in a recent analysis of loci identified in IA, AAA, and TAA, the 11q24 locus is a relatively large block with many genes, none of which are currently implicated in disease pathophysiology.12 The next step in the pursuit of these results will be to perform dense single nucleotide polymorphism genotyping and perform association analyses to narrow the critical interval and identify specific candidate genes.
The chromosome 6p23 region has not previously been linked to IA, AA, or other aneurysmal disease. There are relatively few genes described in this region, although they include the jumonji AT-rich interactive domain 2 associated with cardiovascular abnormalities in mouse models and dystrobrevin binding protein (DTNBP1) associated with a subtype of albinism. The evidence for linkage here is merely suggestive; until confirmed in an independent study, this result should be viewed as preliminary and hypothesis-generating.
The chromosome 9p21 locus that has previously been associated with coronary disease and type 2 diabetes was recently associated with both IAs and AAAs with a population-attributable risk of approximately 26% in those of European decent for each condition.18 These findings, validated in several populations, certainly support the idea of shared genetic risk, although these analyses looked at IA and AAA as separate phenotypes. Similarly, other candidates related to smooth muscle contractility and function have been identified in individuals with familial TAAs13,19 and therefore might be plausible candidates in the broader phenotype. We did not find evidence of linkage to these loci in the current analysis nor was there evidence of linkage in the genomewide linkage analysis of the FIA population overall5 or in a recent meta-analysis of other genomewide linkage studies in IA17 supporting heterogeneity in genetic determinants of these complex disorders. Further specific investigations into these candidate genes may be warranted in this population.
Teasing out shared and distinct genetic risk for IA and AA may have significance for therapeutics as well as risk stratification. In a recent analysis of the role of matrix metalloproteinases in the pathophysiology of aortic and intracranial aneurysm leading to progressive expansion, mRNA and protein expression data pointed to opposite effects of the renin angiotensin system in these 2 diseases.20 Animal data suggest that blockade of matrix metalloproteinases ameliorates expansion but cannot reduce IA formation. They propose different pathological evolution and distinct responses to drug intervention.
The current analysis has several strengths and limitations. The FIA study represents the largest collection of IA pedigrees and data were systematically collected on all aneurysm types. Despite these strengths, the number of pedigrees with both IAs and AAs is relatively small limiting our ability to explore the role of known risk factors such as hypertension and smoking.
Summary
Although the FIA study is the largest study to date of multiplex IA pedigrees, in this report, we have analyzed only a subset of the families defined as those having members with AA. In analyses in the 26 families meeting this criterion, we detected evidence of possible linkage to chromosomes 11 and 6. Analyses of these same families, when limiting the phenotype to only IA, produces some possible evidence of linkage to both regions, but the LOD score on both chromosomes is substantially reduced. We interpret these data to suggest that in these 2 chromosomal regions, gene(s) are located that increase the risk for IA as well as AA. Analyses are underway to evaluate the role of potential candidate genes.
Supplementary Material
Acknowledgments
We acknowledge the diligent work of the local sites that played a crucial role in characterizing the pedigrees and phenotyping family members.
Sources of Funding This study was funded by a grant from the National Institute of Neurological Diseases and Stroke (NINDS RO1 NS39512). Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, Contract Number N01-HG-65403. All of the DNA samples were extracted and prepared for CIDR genotyping in the laboratory of Professor Ranjan Deka at the Center for Genome Information of the University of Cincinnati College of Medicine.
Appendix
Coordinating Center: University of Cincinnati—J. Broderick, Principal Investigator (PI); D. Kleindorfer, coinvestigator; L. Sauerbeck, study coordinator; S. Ewing, administrator; J. Sester, research assistant. Genotyping Center: University of Cincinnati—R. Deka, PI. Linkage Analysis: Indiana University—T. Foroud, PI; D. Koller, coinvestigator; M. Daubs, data manager; J. Gray, research coordinator; D. Lai, statistician. Imaging Center: Mayo Clinic—J. Huston III, Co-PI; D. Kallmes, study neuroradiologist; M. Maronie Smith, MRI study coordinator. Clinical Centers: University of Alabama at Birmingham—W. Fisher, PI; H. Forson, coordinator; Clinical Trials Research Unit, University of Auckland and Auckland City Hospital, New Zealand—C. Anderson, PI, E. Mee, PI, C. Howe, coordinator, S. Vos, coordinator; Royal Perth Hospital, Sir Charles Gairdner Hospital, Royal Adelaide Hospital, Royal Melbourne Hospital, Alfred Hospital, Westmead Hospital, Royal North Shore Hospital, Royal Prince Alfred Hospital, Australia—C. Anderson, PI, G. Hankey, PI, N. Knuckey, PI, J. Laidlaw, PI, P. Reilly, PI, N. Dorsch, PI, M. Morgan, PI, M. Besser, PI, J. Rosenfeld, PI, K. Athanasiadis, coordinator, A. Claxton, coordinator, V. Dunne, coordinator, J. Griffith, coordinator, J. Davidson, coordinator, S. Pope, coordinator, Amanda Froelich, coordinator; Brigham & Women’s Hospital—A. Day, PI, R. Brach, coordinator; University of Cincinnati—D. Woo, Co-PI, M. Zuccarello, Co-PI, A. Ringer, Co-PI, H. Yeh, Co-PI, K. Franklin, coordinator; Cleveland Clinic Foundation—P. Ramussen, PI, D. Andrews-Hinders, coordinator, T. Wheeler, coordinator; Columbia University—E. S. Connolly, PI, R. Sacco, Co-PI, D. LaMonica, coordinator; University of Florida—S. B. Lewis, PI, A. Royster, coordinator; Indianapolis Neurosurgical Group—T. Payner, PI, N. Miracle, coordinator; Johns Hopkins—K. Murphy, PI, B. Kohler, coordinator; Massachusetts General Hospital—C. Ogilvy, PI, D. Buckley, coordinator, J. Manansala, coordinator; London Health Science Center Research Inc—G. Ferguson, PI, C. Mayer, coordinator, J. Peacock, coordinator; Notre Dame Hospital—G. Rouleau, PI, A. Desjarlais, coordinator; University of Maryland—E. F. Aldrich, PI, C. Aldrich, coordinator, C. Byard, coordinator; Mayo Clinic—R. D. Brown, PI, L. Jaeger, coordinator; University of Michigan—L. Morgenstern, PI, M. Concannon, coordinator; New Jersey Medical School—A. I. Qureshi, PI, P. Harris-Ln, coordinator; Northwestern University—H. Batjer, PI, G. Joven, S. Thompson, coordinator; University of Ottawa—M. T. Richard, PI, A. Hopper, PI; University of Pittsburgh—A. B. Kassam, PI, K. Lee, coordinator; University of California, San Francisco—S. C. Johnston, PI, K. Katsura, coordinator; University of Southern California—S. Giannotta, PI, D. Fishback, coordinator; Stanford University Medical Center—G. Steinberg, PI, D. Luu, coordinator, M. Coburn, coordinator; University of Texas at Houston—M. Malkoff, PI, A. Wojner, coordinator; University of Virginia—N. F. Kassel, PI, B. B. Worrall, Co-PI, G. Radakovic, coordinator; University of Washington—D. Tirschwell, PI, P. Tanzi, coordinator; Washington University—C. Derdeyn, PI, M. Catanzaro, coordinator; University of Manitoba (Winnipeg)—A. Kaufmann, PI, D. Gladish, coordinator.
Footnotes
Disclosures
B.B.W. is funded in part by NINDS K08-NS045802.
Some of the data included in this article was presented in February 2008 at the 34th International Stroke Conference, New Orleans, La.
References
- 1.Sandford RM, Bown MJ, London NJ, Sayers RD. The genetic basis of abdominal aortic aneurysms: a review. Eur J Vasc Endovasc Surg. 2007;33:381–390. doi: 10.1016/j.ejvs.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Nahed BV, Bydon M, Ozturk AK, Bilguvar K, Bayrakli F, Gunel M. Genetics of intracranial aneurysms. Neurosurgery. 2007;60:213–225. doi: 10.1227/01.NEU.0000249270.18698.BB.; discussion 225–226.
- 3.Kim DH, Van Ginhoven G, Milewicz DM. Familial aggregation of both aortic and cerebral aneurysms: evidence for a common genetic basis in a subset of families. Neurosurgery. 2005;56:655–661. doi: 10.1227/01.neu.0000156787.55281.53.; discussion 655–661.
- 4.Broderick JP, Sauerbeck LR, Foroud T, Huston J, III, Pankratz N, Meissner I, Brown RD., Jr. The Familial Intracranial Aneurysm (FIA) study protocol. BMC Med Genet. 2005;6:17. doi: 10.1186/1471-2350-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foroud T, Sauerbeck LR, Brown RD, Jr, Anderson C, Woo D, Kleindorfer D, Flaherty ML, Deka R, Hornung R, Meissner I, Bailey-Wilson JE, Rouleau G, Connolly ES, Lai D, Koller DL, Huston J, III, Broderick JP. FIA Study Investigators. Genome screen to detect linkage to intracranial aneurysm susceptibility genes: the Familial Intracranial Aneurysm (FIA) study. Stroke. 2008;39:1434–1440. doi: 10.1161/STROKEAHA.107.502930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehnke M, Cox NJ. Accurate inference of relationships in sib-pair linkage studies. Am J Hum Genet. 1997;61:423–429. doi: 10.1086/514862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannu H, Tran-Fadulu V, Milewicz DM. Genetic basis of thoracic aortic aneurysms and aortic dissections. Am J Med Genet C Semin Med Genet. 2005;139:10–16. doi: 10.1002/ajmg.c.30069. [DOI] [PubMed] [Google Scholar]
- 8.Norrgard O, Angqvist KA, Fodstad H, Forssell A, Lindberg M. Co-existence of abdominal aortic aneurysms and intracranial aneurysms. Acta Neurochir (Wien) 1987;87:34–39. doi: 10.1007/BF02076012. [DOI] [PubMed] [Google Scholar]
- 9.Albright LA Cannon, Camp NJ, Farnham JM, MacDonald J, Abtin K, Rowe KG. A genealogical assessment of heritable predisposition to aneurysms. J Neurosurg. 2003;99:637–643. doi: 10.3171/jns.2003.99.4.0637. [DOI] [PubMed] [Google Scholar]
- 10.Triantafyllidi H, Rizos I, Arvaniti C, Stefanadis C. Incidental aneurysms of aorta and basilar artery in patients with coronary artery ectasia. A magnetic resonance angiography study. Acta Cardiol. 2005;60:619–623. doi: 10.2143/AC.60.6.2004934. [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Akutsu K, Tamori Y, Sakamoto S, Yoshimuta T, Hashimoto H, Takeshita S. Differences in atherosclerotic profiles between patients with thoracic and abdominal aortic aneurysms. Am J Cardiol. 2008;101:696–699. doi: 10.1016/j.amjcard.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Ruigrok YM, Elias R, Wijmenga C, Rinkel GJ. A comparison of genetic chromosomal loci for intracranial, thoracic aortic, and abdominal aortic aneurysms in search of common genetic risk factors. Cardiovasc Pathol. 2008;17:40–47. doi: 10.1016/j.carpath.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan CJ, Casey M, He J, Veugelers M, Henderson K, Guo D, Campagna R, Roman MJ, Milewicz DM, Devereux RB, Basson CT. Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation. 2001;103:2469–2475. doi: 10.1161/01.cir.103.20.2469. [DOI] [PubMed] [Google Scholar]
- 15.Ozturk AK, Nahed BV, Bydon M, Bilguvar K, Goksu E, Bademci G, Guclu B, Johnson MH, Amar A, Lifton RP, Gunel M. Molecular genetic analysis of two large kindreds with intracranial aneurysms demonstrates linkage to 11q24-25 and 14q23-31. Stroke. 2006;37:1021–1027. doi: 10.1161/01.STR.0000206153.92675.b9. [DOI] [PubMed] [Google Scholar]
- 16.Onda H, Kasuya H, Yoneyama T, Takakura K, Hori T, Takeda J, Nakajima T, Inoue I. Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. Am J Hum Genet. 2001;69:804–819. doi: 10.1086/323614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biros E, Golledge J. Meta-analysis of whole-genome linkage scans for intracranial aneurysm. Neurosci Lett. 2008;431:31–35. doi: 10.1016/j.neulet.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jaaskelainen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsater A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemela M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 19.Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, Vick GW, Marian AJ, Raman CS, Buja LM, Milewicz DM. Myh11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin ii. Hum Mol Genet. 2007;16:2453–2462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Ji WJ, Tu Y, Yao M, Li YM. Abdominal aortic aneurysm and cerebral aneurysm present different pathological evolutions and responses to pharmacological therapy. Med Hypotheses. 2007;68:601–606. doi: 10.1016/j.mehy.2006.06.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.