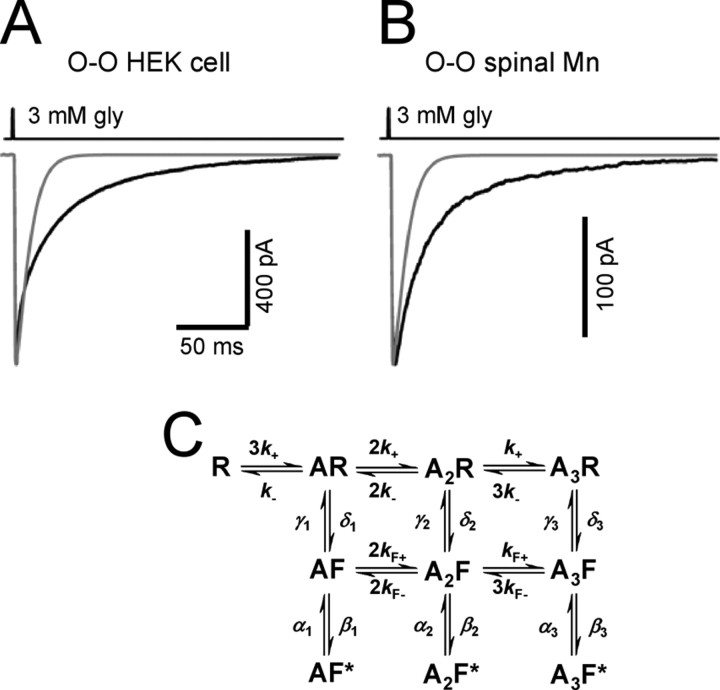
Figure 1.
Glycinergic currents deactivate slowly in outside-out patches. The black traces in A and B are current responses to a 1 ms pulse of 3 mm glycine recorded from outside-out patches from HEK293 cell expressing rat recombinant α1β glycine receptors (A, average of 25 sweeps) or from a P10 juvenile rat motoneuron (B, average of 17 sweeps). The time course of glycine concentration (taken from the open tip potential recorded at the end of the experiment) is shown above each trace, and the holding potential was −100 mV. The gray line superimposed in A and B is the current predicted from cell-attached single-channel kinetic rates (Table 2) for the flip model shown in C for the same glycine pulse (1 ms, 3 mm). The flip scheme (adapted from Burzomato et al., 2004) has three equivalent binding sites. Each of the bound resting states R can switch to a flip conformation F, in which the pore is still shut, but agonist affinity (for all sites) is higher than for R states. The channel can open (to F* states) only from the flipped states. The scheme was fitted with the constraint that the affinity for the three binding sites remains the same as long as the protein stays in the same conformation (either shut or “flipped”), regardless of how many other binding sites are already occupied.