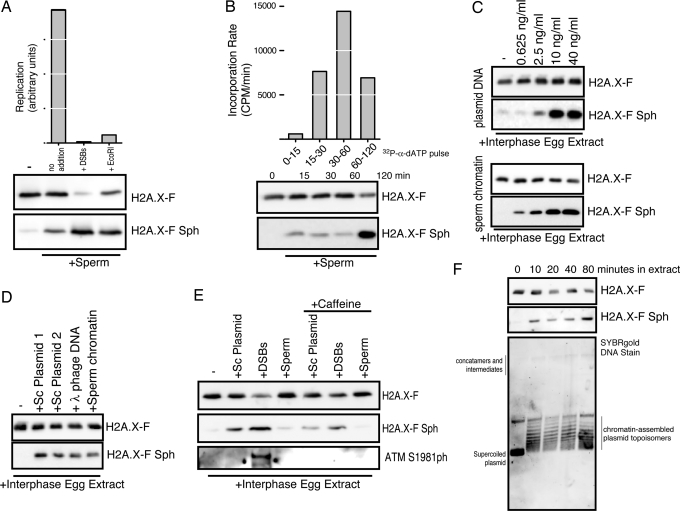
Fig. 5.
H2A.X-F phosphorylation occurs in nondamaged and checkpoint contexts in Xenopus egg extracts. (A) DNA replication of sperm chromatin at 4,000/μL in interphase egg extract with and without DNA damage in trans (DSBs) and damage in cis (EcoRI restriction enzyme) was monitored after 60 min and plotted in arbitrary units. Total extract (0.25 μL) from each condition, incubated in parallel, was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (B) DNA replication of sperm chromatin at 4,000/μL in interphase egg extract was pulse-labeled with 32P-α-dATP for the time periods indicated (0–15, 15–30, 30–60, and 60–120 min). The quantitated incorporation divided by the time produced the plotted rate of incorporation. Total extract (0.25 μL) from each time point, incubated in parallel, was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (C) (Upper) Supercoiled plasmid DNA (≈5 kb pC1-EYFP) was titrated into clarified egg extract and incubated for 60 min. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (Lower) Sperm chromatin was titrated into clarified egg extract and incubated for 60 min. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (D) Supercoiled plasmids pC1-EYFP and pG5ML, lambda phage, sperm chromatin, and DSBs at 40 ng/μL final concentration were incubated in clarified egg extract. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F and phosphorylated H2A.X-F. (E) Supercoiled plasmid DNA, HaeIII-digested plasmid (DSBs), and sperm chromatin at 40 ng/μL final concentration were incubated in clarified egg extract. Total extract (0.25 μL) from each condition was immunoblotted for H2A.X-F, phosphorylated H2A.X-F, and ATM phosphorylated on Ser-1981. (F) Supercoiled plasmid was incubated in clarified egg extract at 40 ng/μL and time points were removed. Isolated DNA was run on an agarose gel at 1.5 V/cm and stained with SYBRgold. Total protein from the same samples was immunoblotted for H2A.X-F and H2A.X-F Sph.