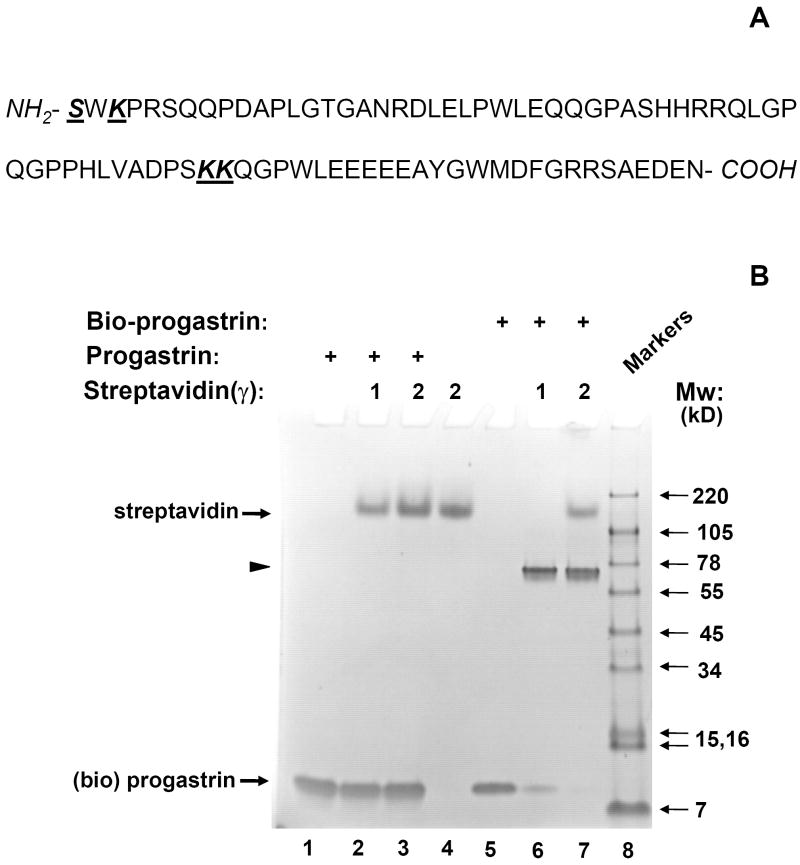
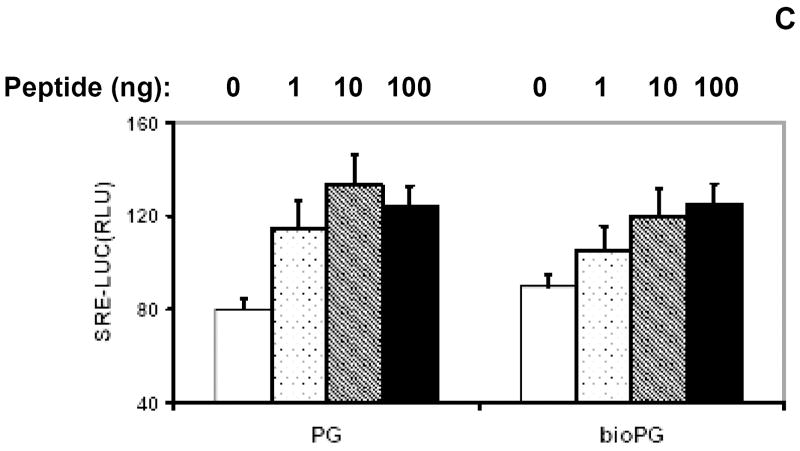
Fig. 1. Effective biotinylation of synthetic progastrin in vitro.
(A). Sequence of human progastrin. Amino acids with free amino groups available for biotinylation, are shown in bold and underlined. (B). The quality of PG biotinylation in samples was tested through analysis of bio-PG: strepavidin complexes in SDS-PAGE electrophoresis. Biotinylated PG (2μg; lanes 5-7) was incubated alone (lane 5) or with 1 or 2 μg of strepavidin (lanes 6, 7) in HBSS for 20 minutes at 20°C prior to electrophoresis. Unmodified PG (2.5μg) was processed in the same manner (lanes 1-3). The control lane (lane 4) contains streptavidin alone (2 μg). At the end of electrophoresis, the gel was stained with “SimplyBlue” reagent (Invitrogen). The migrating positions of the primary reagents are shown by arrows on the left. Filled arrowhead indicates the unique bio-PG: streptavidin complex. The positions of molecular weight markers (Mw; kD) are shown by arrows on the right. (C) Unmodified and biotinylated progastrin peptides stimulate the growth-responsive reporter (luciferase) activity in IEC-18 cells. The cells were transiently co-transfected with growth-factor responsive pSREx5-LUC and reference Renilla-Luciferase plasmids, serum-starved and treated or not with 0, 1, 10, 100nM of PG or bio-PG (empty, dotted, hatched and filled bars, respectively) for 20 hours. Relative (RLU) reporter-to-reference signals were determined for each sample and shown on the abscissa axis. Data are representative of two experiments that were performed.