Table 5.
Bond dissociation energy of the solvents used for the photolysis of halogenated phenothiazines and the TCA-chlorine bond (E[H•] = −0.500273 hartree; E[Cl•] = −460.136242 hartree; 1 Hartree = 627.5095 kcal/mol).
| R-X | E (UBLYP) Hartrees | BDE (kcal/mol) | ||
|---|---|---|---|---|
| R-X | R• | This work | Exp.† | |
| CH3O–H | −115.714405 | −115.050462 | 102.7 | 102 |
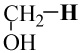 |
−115.714405 | −115.062993 | 94.8 | 92 |
| CH3CH2O–H | −155.034287 | −154.370492 | 102.6 | 103 |
 |
−155.034287 | −154.375316 | 99.6 | - |
| CH3CH2CH2O–H | −194.348026 | −193.685044 | 102.1 | 103 |
 |
−194.348026 | −193.688468 | 100.0 | -- |
 |
−194.353452 | −193.688795 | 103.2 | 103 |
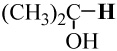 |
−194.353452 | −193.697975 | 97.4 | 94 |
| (CH3)3CO—H | −233.670958 | −233.006172 | 103.2 | 102 |
| −233.670958 | −232.997898‡ | 108.4 | -- | |
| PH-Cl | −1375.239118 | −914.955531 | 92.5 | -- |
| 3(PH-Cl)* | −1375.140165 | −914.955531 | 30.4 | -- |
| PZ-Cl | −1627.126232 | −1166.842675 | 92.4 | -- |
| 3(PZ-Cl)* | −1627.027190 | −1166.842675 | 30.3 | -- |
Values from references (28–31).
Calculated for the beta hydrogen.
