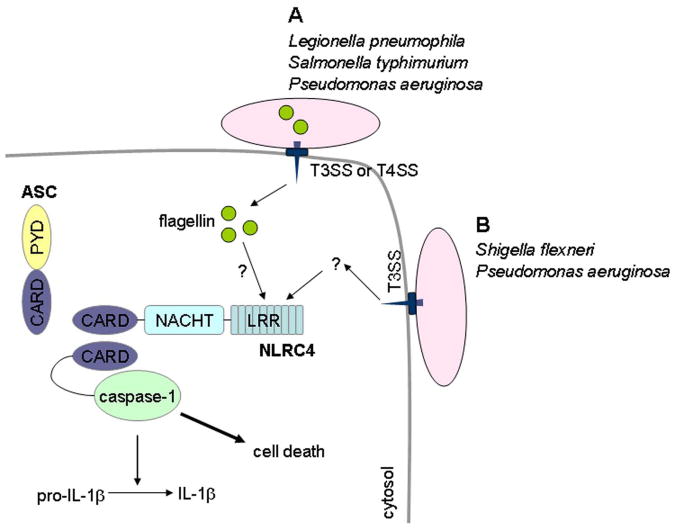
Figure 1.
Activation of the NLRC4 inflammasome by Gram negative bacteria. Activation of caspase-1 following infection of macrophages with S. typhimurium, P. aeruginosa, S. flexneri or L. pneumophila requires a functional type III or type IV secretion system. Infection causes NLRC4 to undergo a conformational change by an unknown mechanism, which allows NLRC4 to oligomerize. Following oligomerization, NLRC4 recruits pro-caspase-1 via homophilic CARD-CARD interactions, which leads to activation of caspase-1. ASC is required for NLRC4-mediated caspase-1 activation although its exact role remains unclear. (A) Bacterial-derived cytosolic flagellin augments caspase-1 activation following infection with L. pneumophila, S. typhimurium, and P. aeruginosa. (B) Activation of caspaspe-1 following infection of macrophages with S. flexneri and P. aeruginosa can occur independently of flagellin.