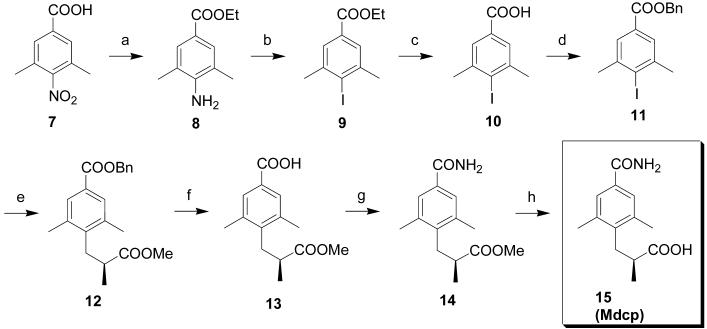
Scheme 1.
Reagents and conditions: (a) EtOH, dry HCl (g), reflux 6 h, followed by Sn powder, 40 °C, 4 h, 82%; (b) NaNO2, conc. HCl, KI, acetone, 83%; (c) LiOH, THF-MeOH-H2O, 0 °C to rt, 95%; (d) K2CO3, BnBr, DMF, rt, 6 h, 92%; (e) Zn-Cu couple, (R)-methyl 3-iodo-2-methylpropanoate, PdCl2[P(o-Tol)3]2, Benzene, 52%; (f) Pd/C, H2(g), MeOH, rt, overnight, 96%; (g) (COCl)2, CH2Cl2, rt, 2 h then NH4OH (25%), 0 °C to rt, 2 h, 87%; (h) LiOH (1N) - THF (1 : 1), 0 °C, 2.5 h, 78%.