Figure 11.
Models of Amylopectin Synthesis in the Wild Type and Aberrant Glucan Synthesis in the Mutant Combinations Studied Here.
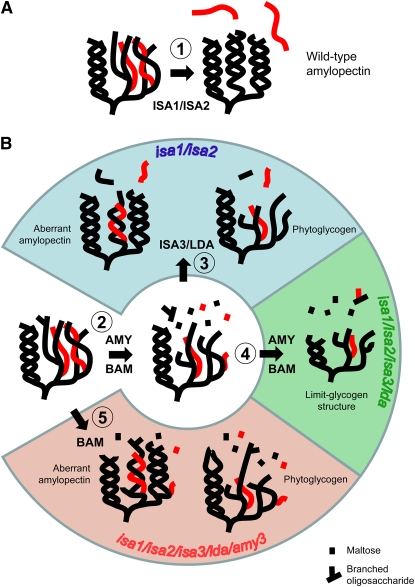
(A) Glucans produced by starch synthases and starch branching enzymes produce a branched amylopectin-like glucan capable of crystallization to form starch granules. This process is accelerated by the ISA1/ISA2 heteromultimeric enzyme (1), which selectively removes wrongly positioned branch points (red).
(B) Glucans produced by starch synthases and starch branching enzymes, as in (A), are not selectively debranched in the absence of ISA1 and/or ISA2, delaying the formation of secondary structures and rendering the glucans susceptible to α- and β-amylolysis. This results in the formation of short chains and liberation of maltose and branched malto-oligosaccharides (2). In the isa1/isa2 double mutant, very short chains can still be removed by ISA3 and/or LDA, enabling some aberrant amylopectin to be synthesized, but the majority of glucans remain soluble as phytoglycogen (3; blue section). Differences in amylolytic capacity may explain why some cells preferentially make starch granules (e.g., epidermal cells) and others make phytoglycogen (mesophyll cells). In the absence of all DBEs, the aberrant glucans cannot be degraded and are susceptible to further amylolysis, resulting in a limit glycogen–like structure (4; green section). In the absence of all DBEs and AMY3, nascent amylopectin produced by starch synthases and starch branching enzymes are subjected to β-amylolysis but not α-amylolysis (5; red section). Reduced amylolytic modification of the glucan allows a greater fraction to crystallize. We predict that removal of BAM proteins would further favor starch granule production. We emphasize that these schemes are simplified and that rather than being a linear or discontinuous process, as indicated, the glucans are likely to be continuously elaborated and degraded during synthesis. However, we propose that the schemes serve as a good basis for further evaluation.