Figure 2.
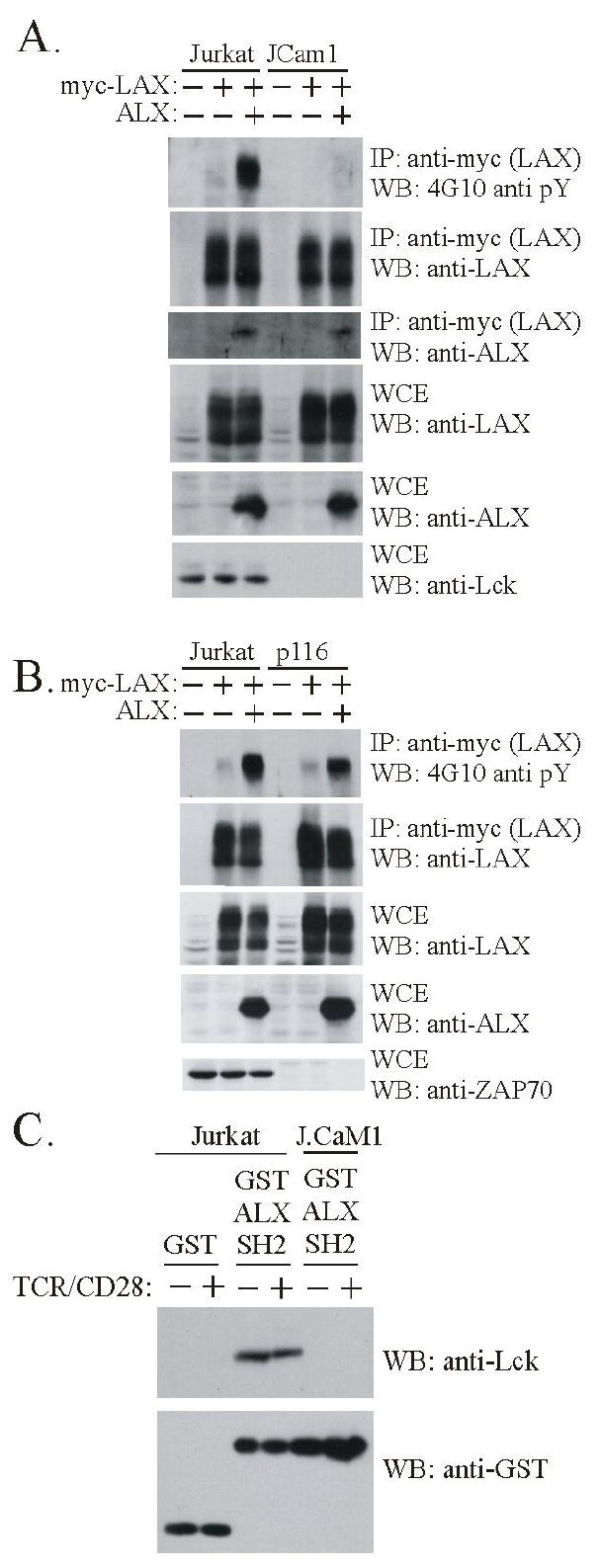
ALX promotes phosphorylation of LAX dependent on the tyrosine kinase Lck. (A) Cells were transfected and subject to immunoprecipitation without TCR stimulation as in Fig. 1 except that, as indicated, Jurkat or J.Cam1 Jurkat mutant (Lck deficient) cell lines were used. Each cell line was transfected with LAX alone or in combination with ALX. Transfection of an expression construct (+) or substitution of empty vector (-) is indicated. Immunoprecipitates and WCE were analyzed by Western blot with antibodies to phosphotyrosine (4G10) to assess LAX phosphorylation, as well as LAX to confirm effective immunoprecipitation, and ALX to confirm association. WCE were analyzed with antibodies to LAX and ALX to assess expression, and against Lck to confirm the deficiencies in JCam1 (B) Examination of the requirement for ZAP-70 in ALX-mediated phosphorylation was examined in p116 ZAP-70 deficient Jurkat T cell line, as was done with JCam1 in part (A) above. (C) Extracts were made from either Jurkat or J.Cam1 cells that were either unstimulated (-) or stimulated for 2 minutes (+) with antibodies to TCR and CD28. Extracts were incubated with either GST or a recombinant protein composed of GST and the SH2 domain of ALX (GST ALX SH2), both bound to beads. The beads were precipitated and washed before being analyzed by western blotting. The anti-Lck blot shows that Lck bound specifically to the SH2 domain of ALX (upper panel). Western blotting with antibodies to GST demonstrates the presence of equivalent amounts of fusion protein in each precipitate (lower panel).
