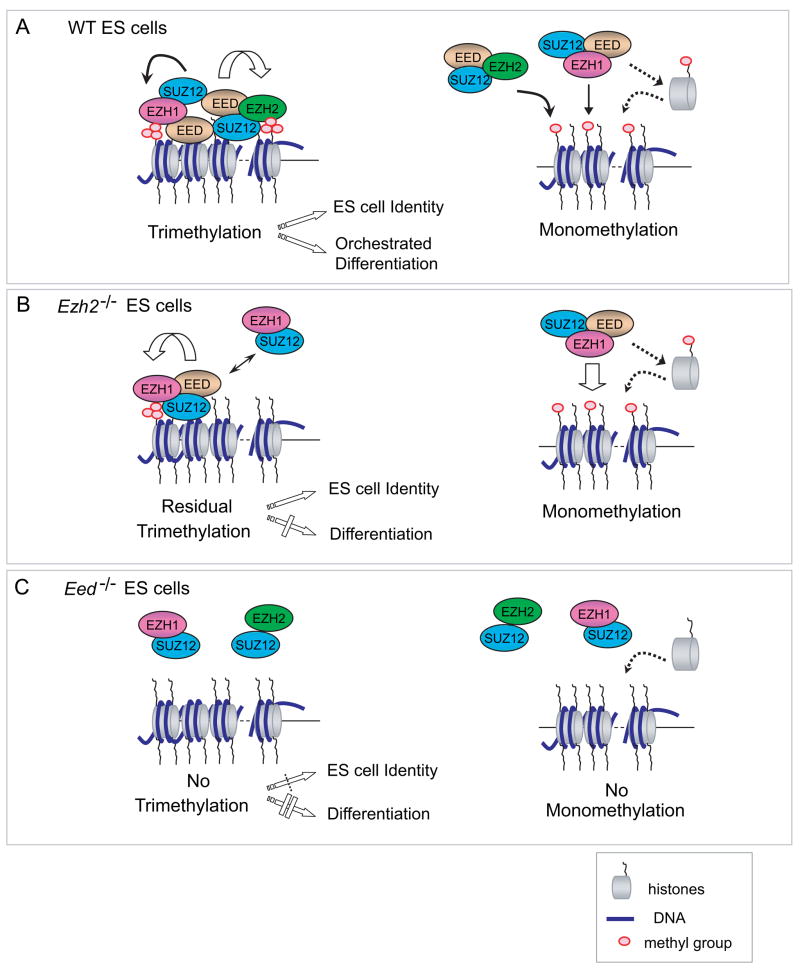
Figure 7. Complementary but non-redundant roles of EZH1 and EZH2 in mediating methylation on H3K27 and ES cell function.
(A) A model in wild-type (WT) ES cells. Both EZH2 and EZH1 bind to EED and SUZ12, forming alternative but interacting PRC2 complexes containing HMTase activity. EZH2-mediated PRC2 is more abundant in ES cells and plays a major role in forming H3K27me3, which is required for ES cell identity and proper differentiation. Both EZH1 and EZH2 containing PRC2 complexes may mediate monomethylation on H3K27 in a non-targeted manner by either acting transiently on nucleosomes across the genome or on free H3 histones prior to nucleosomal assembly during DNA replication.
(B) In Ezh2−/− ES cells, EZH1-PRC2 mediates monomethylation and residual trimethylation on H3K27. EED may regulate EZH1-PRC2 complex in an EZH2-dependant manner. EZH1 mediates H3K27me3 on a set of developmental genes and prevents derepression of PRC2 target genes from globe loss of the H3K27me3 mark in Ezh2−/− ES cells. Hereby, EZH1 complements EZH2 in the maintenance of ES cell identity. However, EZH1-PRC2 cannot fully rescue defects in mesoendodermal development during Ezh2−/− cell differentiation, suggesting non-redundant functions of these two PRC2 complexes in lineage commitment.
(C) Eed−/− ES cells lack all levels of methylation on H3K27, indicating the essential role of EED in EZH1 or EZH2 mediated PRC2 complexes. Complete loss of H3K27me3 in the absence of Eed confounds ES cell identity by de-repressing a set of differentiation-regulated PRC2 target genes, and blocks the proper execution of pluripotent programs.