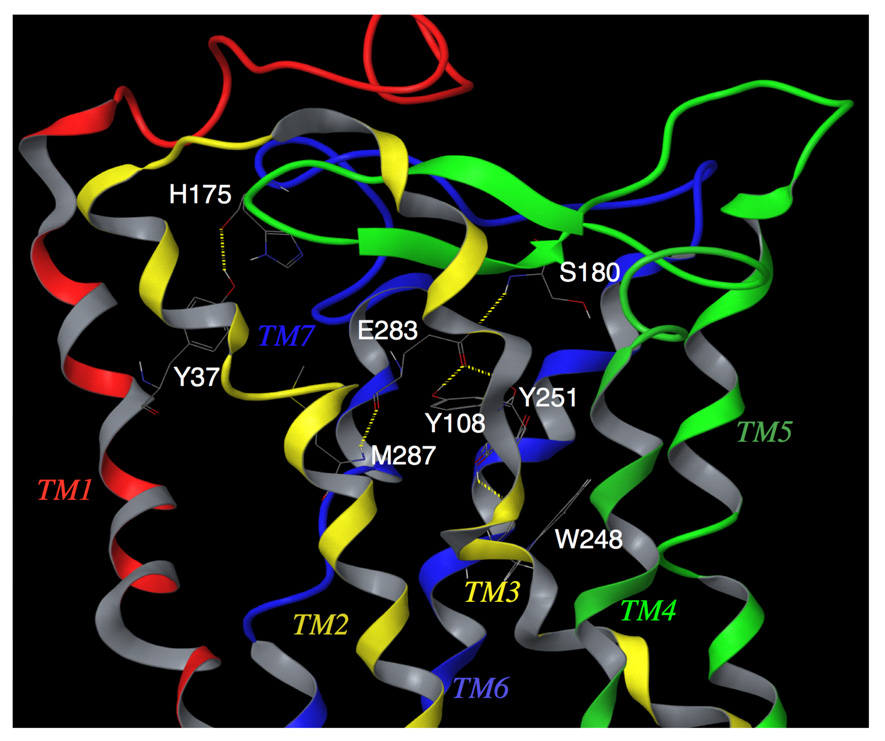
Fig. 3.
Transmembrane residues Y108(TM3) / E283(TM7) / Y251(TM6) / M287(TM7) / S180(ECL2) of unliganded CCR5, forming a hydrogen bond network. Y37 has hydrogen bond interaction with H175. This shows that transmembrane residues, implicated in CCR5 inhibitor binding, have direct interactions with ECL2 residues in the unliganded receptor. It is assumed that these intramolecular interactions are responsible for maintaining a conformation of ECL2 that is favorable for binding with HIV-1 gp120/CD4 complex. AK530 interacts with Y37, Y108, E283, and Y251 in the binding cavity and disrupts the intramolecular interactions of Y108 and Y251 with E283, and those of E283 with the ECL2 (Fig. 5). It is assumed that these structural changes, after inhibitor binding, alter the conformation of the ECL2, resulting in loss of association with gp120. The analysis strongly suggests that the loss of hydrogen bonds between helices may cause allosteric conformational changes in ECL2 leading to the inhibition of HIV-1 gp120 binding to CCR5.