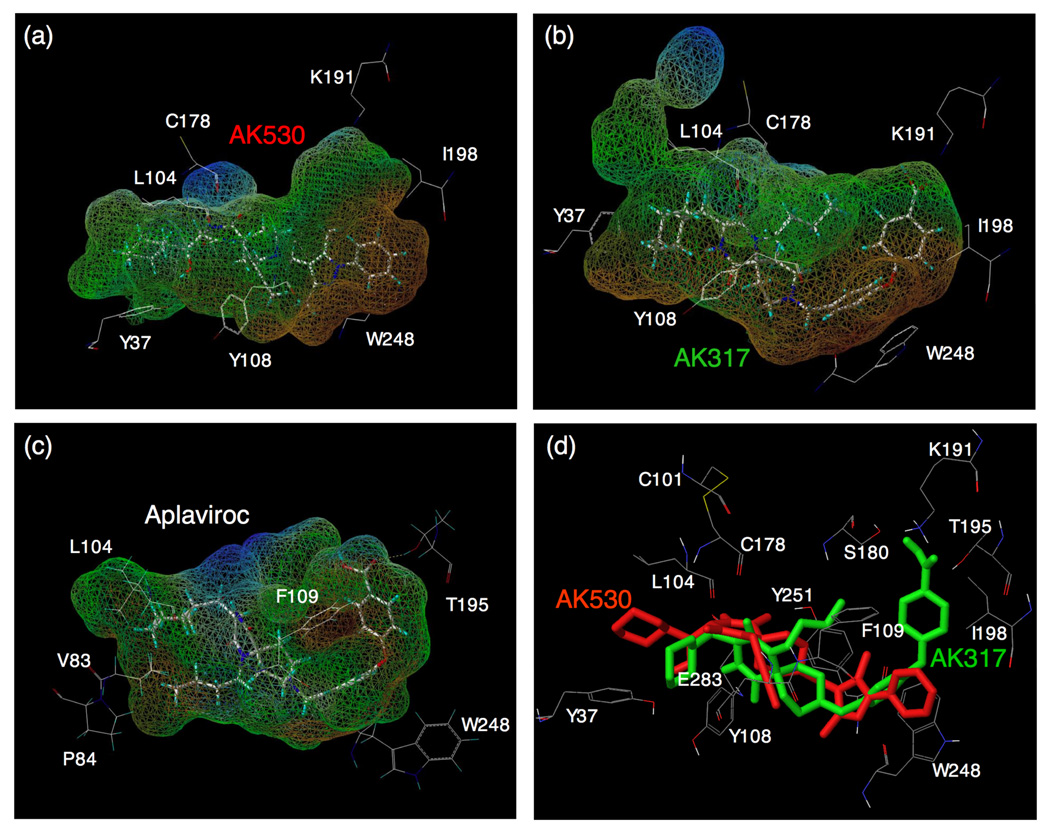
Fig. 4.
Lipophilic potential mapped on the binding cavity of CCR5 inhibitors. The lipophilic potential mapped onto the binding cavity when AK530, AK317, and APL bind to CCR5 are shown in (a), (b), and (c), respectively. The predominantly lipophilic region of the cavity is shown in brown (bottom region of the cavity which is towards the cytoplasmic region of CCR5). The blue regions are predominantly hydrophilic (present towards the extra-cellular region), and the green regions are moderately lipophilic. The figure was generated using MOLCAD. The shape of the binding cavity is slightly different for AK530 and AK317 as the receptor conformations are slightly different when these molecules bind to CCR5. The unoccupied volumes of the cavities suggest optimization ideas for improving the potency of these molecules. (d) The binding modes of AK530 and AK317 superimposed. AK530 is shown in red and AK317 in green. Note that the binding orientation in the vicinity of TM helices 5 and 6 differ. AK317 binds towards and around ECL2 residues, whereas AK530 bends towards the intracellular domain. AK530 has a high binding affinity probably because it binds “deeper” into the cavity. On the other hand, by being able to interact with ECL2 residues, AK317 maintains comparable anti-HIV-1 potency with AK530 even though its binding affinity is about ten fold lower.