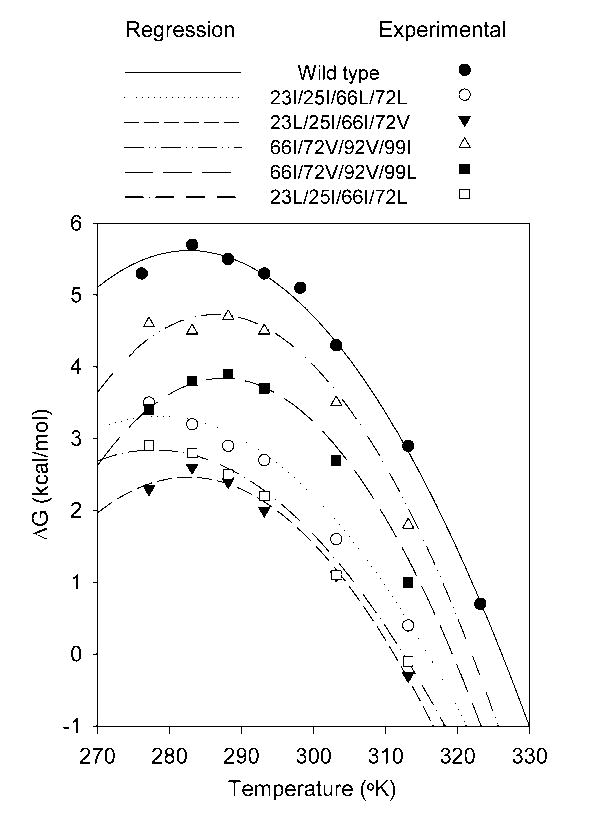
Figure 1.
Plot of ΔGH2O against temperature for wild-type staphylococcal nuclease and five representative quadruple mutants. The mutant 23I/25I/66L/72L was chosen as it has a low ΔCp and subjectively amongst the worst fits. The mutant 23L/25I/66I/72V has a markedly lower stability than wild-type, yet has a ΔCp that is nearly the same and a subjectively good fit. The mutants 66I/72V/92V/99I and 66I/72V/92V/99L are poor and good fits respectively but both with high ΔCp. The mutant 23L/25I/66I/72L has a low ΔCp and a good fit. The lines are the best fit against the Gibbs-Helmholtz equation obtained by non-linear regression of predicted ΔG against ΔGH2O for all measured temperatures while defining Tm and ΔH as the values obtained from thermal denaturation experiments. The points indicate experimental data obtained at various temperatures. The standard deviation in ΔGH2O found in repeated determinations of wild-type stability at 20°C is ± 0.1 kcal/mol.