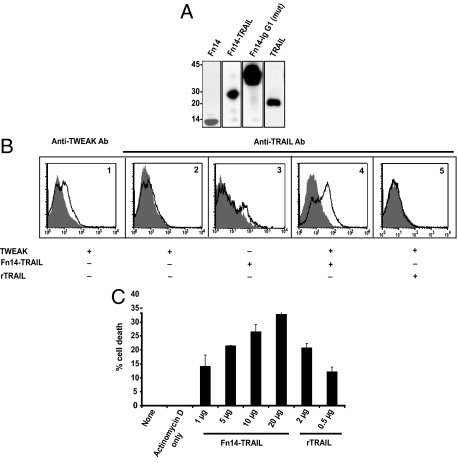
Figure 1.
Expression and functional analysis of Fn14-TRAIL. A: Western blot analysis was performed on conditioned media from 293 cells transfected with expression constructs for Fn14, Fn14-TRAIL, Fn14-IgG1(mut), and TRAIL. Observed bands were consistent with the expected sizes of 8.7 kDa, 27.5 kDa, 34.1 kDa, and 19.0 kDa, respectively. B: CHO cells were transiently transfected with a murine TWEAK cDNA expression construct, and after 48 hours, were incubated at 4°C with purified Fn14-TRAIL or rTRAIL in sodium azide-containing buffer. The presence of membrane-bound TWEAK on transfectants, and the binding of Fn14-TRAIL to them, was verified by flow cytometry, using anti-mouse TWEAK Ab (B1) and anti-mouse TRAIL Ab (B2–B5) as detecting Ab, respectively. B1: TWEAK is expressed on transfected CHO cells, as detected using anti-TWEAK Ab (solid black line) versus isotype control (filled histogram). B2: TRAIL is not detectable on CHO cells, when analyzed using anti-TRAIL Ab (solid black line) versus isotype control (filled histogram). B3 and B4: TRAIL epitopes are enhanced when Fn14-TRAIL is added to TWEAK-expressing, as opposed to TWEAK-negative, CHO cells. Anti-TRAIL Ab and isotype control are represented by solid black line and filled histogram, respectively. B5: TWEAK-transfected cells do not bind to anti-TRAIL Ab (solid black line) in the presence of rTRAIL. Isotype control is shown as filled histogram. C: L929 cells were cultured in flat-bottomed 96-well plates at 2 × 104 cells/well, in 100 μl of AIM-V medium. Sixteen hours later, actinomycin D was added to the cultures at 1 mg/well, and cells were cultured for another 2 hours. Fn14-TRAIL or rTRAIL, as positive control, was then added, and cultures were maintained for an additional 5 hours. The percentage of dead cells was determined by an MTT assay, as described in the Materials and Methods.