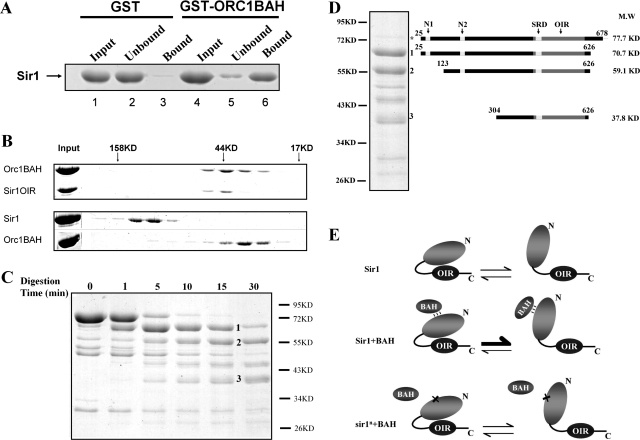
FIG. 7.
Sir1 is less effective than Sir1OIR in interacting with the Orc1BAH in vitro. (A) Recombinant full-length Sir1 (amino acids 25 to 678) purified from E. coli bound a GST-Orc1BAH fusion protein. (B) Gel filtration was used to compare the strength of a Sir1OIR-Orc1BAH interaction (top) to that of a full-length Sir1-Orc1BAH interaction (bottom). Fractions that eluted from the gel filtration column were examined by SDS-PAGE. Gels were stained with Coomassie blue. The elution positions of molecular mass standards on the gel filtration column are indicated at the top of the gel. (C) Purified Sir1 was subjected to limited proteolysis by trypsin at room temperature. Aliquots of Sir1 reaction mixtures incubated in the absence of trypsin for 30 min (time zero) or in the presence of limiting amounts of trypsin for the indicated times were analyzed by SDS-PAGE. (D) The N termini of the three major trypsin fragments generated in panel C (numbered) were identified by Edman degradation sequencing. The C-terminal amino acid was deduced based on the fragment size and the positions of trypsin cleavage sites. The fragment indicated by an asterisk was full-length protein that remained after the 15-min limited trypsin digest. The N1, N2, SRD, and OIR are indicated in the diagram of Sir1 at the top. (E) A speculative model to explain how the N-terminal region of Sir1 could modulate the Sir1OIR-Orc1BAH interaction. Sir1 exists in two different conformations that can interconvert. One of these conformations (left) masks the Sir1OIR and prevents it from binding to the Orc1BAH domain. However, a weak and/or transient interaction between the Sir1 N terminus and a BAH domain (Sir1+BAH) could favor formation of the Sir1 conformation in which the Sir1OIR is fully exposed for a high-affinity interaction with Orc1BAH. In the two-hybrid and in vitro experiments, the BAH domain that weakly interacts with the Sir1 N terminus is Orc1BAH, but in vivo the putative BAH domain is unknown. Mutations that affect the N-terminal region of Sir1 might be defective in silencing and in the two-hybrid interaction with Orc1BAH in part because they disrupt a weak Sir1N-BAH interaction (sir1n+BAH), leading to a reduction in the levels of Sir1 in the conformation that presents the Sir1OIR for a high-affinity interaction with Orc1BAH. Overexpression of such a mutant might provide enough of the open-conformation form of Sir1 needed for silencing. Note that this abstract model attempts to address only the paradoxical Sir1-Orc1BAH interaction dynamics reported in this study. It is probable, based on measurements of silencing and Sir1 binding to the silencer, that the Sir1 N-terminal region plays a role in stabilizing Sir1 after it has established its interaction with ORC.