Figure 4. Interactions between Coronin 1B and Slingshot 1L.
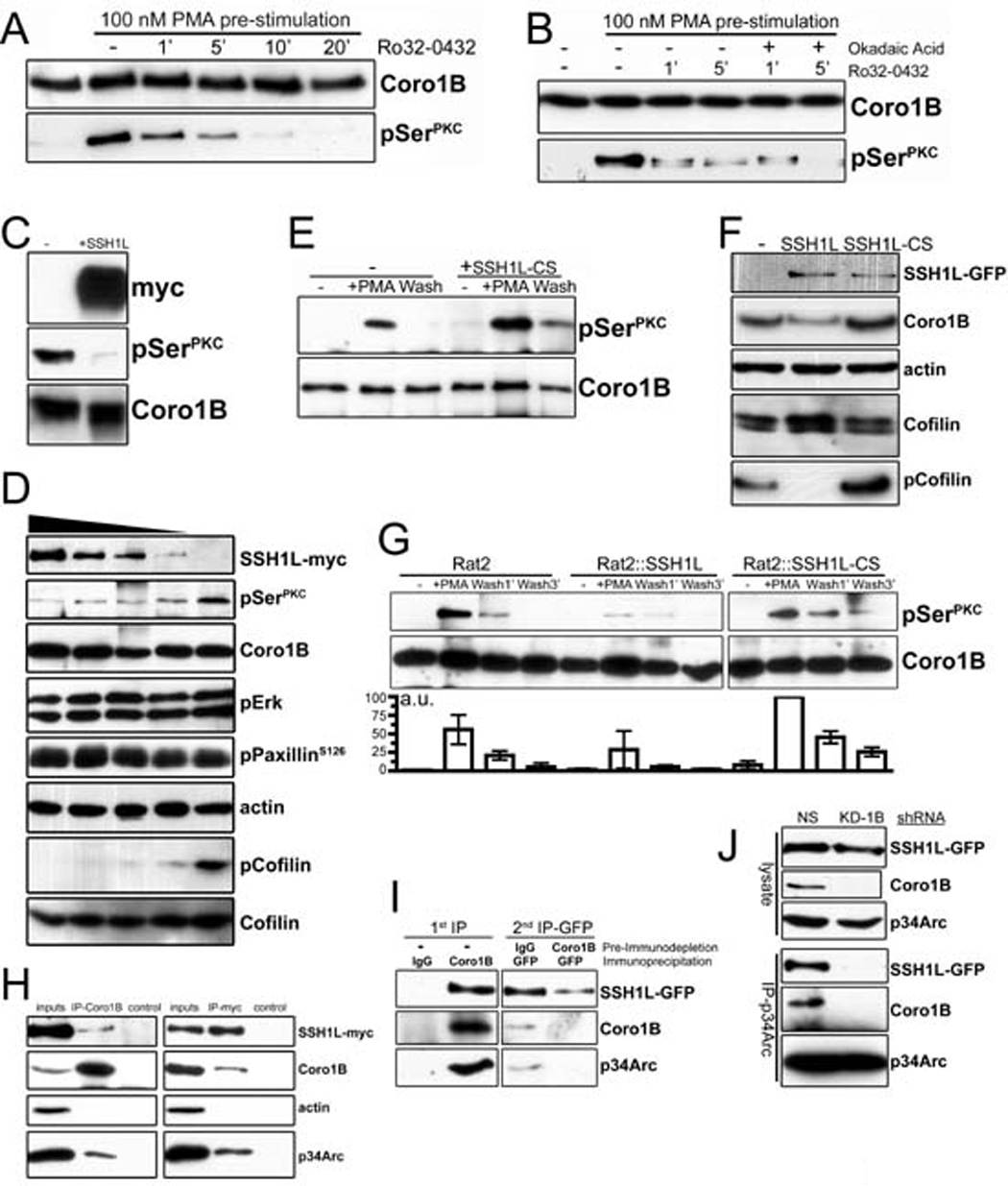
A- Rat2 fibroblasts stably expressing Coronin 1B-GFP were treated with 100 nM PMA for 30 min to stimulate Coronin 1B phosphorylation. Cells were washed with fresh media and incubated with the pan-PKC inhibitor Ro32-0432 (1 µM) to inhibit further phosphorylation. Coronin 1B was immunoprecipitated from lysates at the indicated times and blotted with anti-pSerPKC to monitor dephosphorylation kinetics.
B- Dephosphorylation assay carried out as in panel A with or without okadiac acid (100 nM).
C- Recombinant Coronin 1B was phosphorylated in vitro by PKC and subjected to in vitro dephosphorylation with SSH1L-myc immunoprecipitated from transfected HEK293 cells. Blots were reacted with anti-pSerPKC to detect phospho-Coronin 1B.
D- Serum starved Rat2 fibroblasts were treated with 100 nM PMA for 30 min and lysates were subjected to dephosphorylation in vitro by increasing concentrations of SSH1L-myc. Phospho-Coronin 1B was detected following immunoprecipitation as described above. Phosphorylation of PaxillinS126, Erk1/2 and Cofilin were monitored by immunoblotting the unbound fraction after Coronin 1B immunoprecipitation using phospho-specific antibodies. Blots shown are representative of at least three independent experiments.
E- Phospho-Coronin 1B was detected in control or HEK293 cells expressing myc-tagged dominant negative mutant SSH1L C393S (CS) as described in panel B.
F- Lysates of control Rat2 cells or cells expressing either WT SSH1L-GFP or SSH1L-CS-GFP were blotted with the indicated antibodies.
G- Rat2 cells stably expressing either WT SSH1L-GFP or SSH1L-CS-GFP were subjected to the in vivo dephosphorylation assay. Phospho-Coronin 1B was detected by anti-pSerPKC and quantified by densitometry relative to total Coronin 1B. Results from three independent experiments are presented as means with error bars indicating standard error of the means.
H- Lysates from HEK293 cells transiently expressing SSH1L-myc were immunoprecipitated with antibodies to endogenous Coronin 1B or SSH1L-myc and blotted with the indicated antibodies. Immunoprecipitations with rabbit or mouse IgG were performed as negative controls.
I- Endogenous Coronin 1B was immunodepleted from lysates of Rat2 cells stably expressing SSH1L-GFP. Control rabbit IgG was used for mock depletion. Residual SSH1L-GFP was immunoprecipitated from the immunodepleted lysates using anti-GFP antibody and blotted with the indicated antibodies.
J- Rat2 cells stably expressing SSH1L-GFP were infected with lentivirus expressing Coronin 1B shRNA (KD-1B) or a control shRNA (NS). Arp2/3 complex was immunoprecipitated using anti-p34Arc and blotted for SSH1L using anti-GFP. Similar results were obtained using anti-GFP to immunoprecitate SSH1L-GFP (not shown).
