Abstract
Sphingosine kinase 1 (SphK1) phosphorylates sphingosine to form sphingosine-1-phosphate (S1P) and is a critical regulator of sphingolipid-mediated functions. Cell-based studies suggest a tumor-promoting function for the SphK1/S1P pathway. Also, our previous studies implicated the SphK1/S1P pathway in the induction of the arachidonic acid cascade, a major inflammatory pathway involved in colon carcinogenesis. Therefore, we investigated whether the SphK1/S1P pathway is necessary for mediating carcinogenesis in vivo. Here, we report that 89% (42/47) of human colon cancer samples stained positively for SphK1, whereas normal colon mucosa had negative or weak staining. Adenomas had higher expression of SphK1 vs. normal mucosa, and colon cancers with metastasis had higher expression of SphK1 than those without metastasis. In the azoxymethane (AOM) murine model of colon cancer, SphK1 and S1P were significantly elevated in colon cancer tissues compared to normal mucosa. Moreover, blood levels of S1P were higher in mice with colon cancers than in those without cancers. Notably, SphK1−/− mice subjected to AOM had significantly less aberrant crypt foci (ACF) formation and significantly reduced colon cancer development. These results are the first in vivo evidence that the SphK1/S1P pathway contributes to colon carcinogenesis and that inhibition of this pathway is a potential target for chemoprevention.—Kawamori, T., Kaneshiro, T., Okumura, M., Maalouf, S., Uflacker, A., Bielawski, J., Hannun, Y. A., Obeid, L. M. Role for sphingosine kinase 1 in colon carcinogenesis.
Keywords: inflammation, azoxymethane, dextran sodium sulfate, cell proliferation, apoptosis
Colorectal cancer is the second leading cause of all cancer-related deaths, causing ∼52,000 annual deaths in the United States (1). Despite aggressive screening guidelines for early detection and detailed knowledge of several critical events underlying the pathogenesis of cancer, it continues to be a major health concern in developed countries.
Sphingolipids, especially ceramide, sphingosine, and sphingosine-1-phosphate (S1P), play key roles as regulatory molecules in cancer development (2). S1P promotes cell proliferation and survival, and regulates angiogenesis, whereas sphingosine and ceramide inhibit cell proliferation and stimulate apoptosis. Sphingosine kinase (SphK) phosphorylates sphingosine to form S1P, and is a critical regulator of sphingolipid-mediated functions. Two mammalian isozymes, SphK1 and SphK2, have been characterized (3). The inducible SphK1 is activated by numerous growth factors and cytokines. The S1P product is crucial in the regulation of a variety of biological processes, including Ca2+ mobilization, cytoskeletal reorganization, and cell growth, differentiation, survival, and motility via acting as an intracellular second messenger and an extracellular ligand for G-protein-coupled receptors (S1P1-5) (4, 5). In several studies, the SphK1/S1P pathway has been implicated in tumorigenesis. Overexpression of human SphK1 in NIH3T3 cells resulted in their acquisition of a transformed phenotype, as determined by assays of focus formation, colony growth in soft agar, and their ability to form tumors in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (6), which suggested that SphK1 acts as an oncogene. These observations implicate the SphK1/S1P pathway in cell growth, transformation, and cancer.
Mechanistic studies suggest a relation of SphK1/S1P to the regulation of cyclooxygenase-2 (COX-2), thus suggesting a connection to COX-2-regulated cancers. Indeed, substantial evidence reveals that the arachidonic acid cascade, especially COX-2 and its product, prostaglandin E2 (PGE2), is involved in colon carcinogenesis. For example, COX-2 inhibitors significantly reduced colon carcinogenesis in rodents (7, 8) and inhibited intestinal polyp formation in familial adenomatous polyposis patients (9). PGE2 administration enhanced colon carcinogenesis (10) and overcame indomethacin-reduced intestinal polyp formation in ApcMin (Min) mice (11). In several recent studies (12,13,14,15), the SphK1/S1P pathway was shown to mediate COX-2 expression and PGE2 production in various cell models of cytokine action. Previously (13), we found that COX-2 expression is mediated by the SphK1/S1P pathway in colon cancer cells: We reported that SphK1 down-regulation by RNA interference (RNAi) significantly reduced COX-2 expression and PGE2 production induced by cytokines in HT-29 human colon cancer cells. Enforced SphK1 expression also induced COX-2 protein in normal intestinal epithelial cells. In addition, we found that SphK1 protein and message levels were increased in azoxymethane (AOM) -induced colon tumors in male F344 rats. However, it was unclear whether the SphK1/S1P pathway is upstream of the COX-2/PGE2 pathway in vivo and whether SphK1 is required for colon carcinogenesis in vivo.
Recently, it was reported that in the Min mouse model of intestinal neoplasia, SphK1 deficiency did not reduce the number of intestinal polyps, but reduced the size of polyps (16), providing evidence that SphK1 is involved in intestinal polyp formation induced by an adenomatous polyposis coli (APC) mutation. The Min mouse model is unique, because most intestinal polyps are observed in the small intestine and very few are observed in the large intestine.
In contrast, AOM is commonly used to induce colonic neoplasia in rodents, and the spectrum of colonic lesions and the genetic events in the AOM-induced colon carcinogenesis model are similar to the various types of neoplastic diseases in the human colon, including the aberrant crypt foci (ACF) adenoma-carcinoma sequence. Moreover, dextran sodium sulfate (DSS) administration to rodents is widely employed to recapitulate human inflammatory bowel diseases (IBDs) such as ulcerative colitis, because it causes an inflammatory reaction and ulceration throughout the entire colon, similar to that observed in IBD patients (17). The combination of AOM/DSS shares most features with human ulcerative colitis and enhances colon carcinogenesis via activation of inflammatory mediators such as COX-2, inducible nitric oxide synthase (iNOS), and β-catenin in colon tumors (18).
In the present study, we provide in vivo evidence for a role for the SphK1/S1P pathway in AOM/DSS-induced colon carcinogenesis. We also show that SphK1 is upregulated in human colon carcinogenesis and that SphK1 knockout (KO) mice were significantly protected in the early and late stages of colon carcinogenesis. We then discuss the implications of these results for the role of the SphK1/S1P pathway and its potential in colon cancer chemoprevention and therapy.
MATERIALS AND METHODS
Human colon samples
Human colorectal tissue microarray slides were obtained from the Cooperative Human Tissue Network (CHTN) of the National Cancer Institute (NCI), U.S. National Institutes of Health (NIH; Bethesda, MD, USA). These tissue microarray slides included 7 cases of normal mucosa from colons resected for diverticulosis, 7 cases of normal mucosa from colons resected for colorectal adenocarcinoma, 14 cases of colorectal adenoma, 14 cases of primary colorectal adenocarcinoma, 7 cases of colorectal adenocarcinomas metastatic to regional lymph nodes, and 7 cases of colorectal adenocarcinomas metastatic to distant organs. Formalin-fixed, paraffin-embedded materials from 19 colorectal adenocarcinomas and adjacent normal colon mucosa tissues were obtained from Tissue Procurement and Bank, Hollings Cancer Center, Medical University of South Carolina (MUSC; Charleston, SC, USA). All samples were selected by a certified surgical pathologist under a protocol approved by the institutional review boards at the MUSC.
Immunohistochemistry
All immunohistochemical analyses were carried out with the avidin-biotin complex immunoperoxidase technique. Tissue sections were mounted on glass slides, deparaffinized with xylene, and rehydrated with graded alcohol. After blocking endogenous peroxidase with 3% hydrogen peroxide in methanol for 10 min, slides were immersed in 10 mM citrate buffer (pH 6) and placed in a steamer for 30 min to enhance antigen exposure. Then, slides were incubated with 2% normal host serum of secondary antibody in PBS for 30 min. Rabbit polyclonal antibodies against human SphK1 (19), and cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA), and a goat polyclonal antibody against COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and a rat monoclonal antibody (Abcam Inc., Cambridge, MA, USA) against 5′-bromodeoxyuridine (BrdU) were used as the primary antibodies for the immunostaining at 1:100 dilution. Biotinylated goat anti-rabbit immunoglobulin G (IgG), rabbit anti-goat IgG, and rabbit anti-rat IgG were secondary antibodies. Immunostaining was developed using Vectastain ABC reagents (Vector Laboratories, Burlingame, CA, USA), 3,3′-diaminobenzidine, and hydrogen peroxide. Tissue sections were counterstained with hematoxylin. As a control, duplicate sections were stained without primary antibodies. Immunohistochemical expression was measured by calculating scores for SphK1 or COX-2 immunostaining in colorectal samples: 0, negative; 1, weak staining in <50% of cells; 2, moderate staining in >50% of cells; 3, strong staining in >50% of cells.
Animal experiments
Mice were housed and handled in the division of laboratory animal resources facility under MUSC guidelines. Mice were maintained under controlled conditions of humidity (50±10%), light (12/12 h light/dark cycle) and temperature (23±2°C). All mouse experiments were approved by the institutional animal care and use committee at MUSC. SphK1 homozygous KO mice of the 129SV-C57BL/6 background, a kind gift from Dr. Richard L. Proia [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/NIH, Bethesda, MD, USA), were backcrossed to C57BL/6 wild-type mice (purchased from Charles River Laboratories, Wilmington, MA, USA) at least 10 times (20). Heterozygous SphK1 and SphK2 KO mice with Balb/c background were kind gifts from the Novartis Institute for BioMedical Research (Vienna, Austria) (21). Genotypes of SphK1 and SphK2 KO mice were determined by polymerase chain reaction (PCR) analysis of genomic DNA isolated from tail biopsies (20, 21).
ACF formation assay
AOM was obtained from the NCI Chemical Carcinogen Reference Standard Repository (Bethesda, MD, USA). Mice received an intraperitoneal (i.p.) AOM injection (10 mg/kg) 1×/wk for 3 wk, starting at 6 wk of age. All animals were sacrificed at 2 wk after the last AOM administration. After laparotomy, the entire colon was resected and filled with 10% neutral buffered formalin and then opened longitudinally from the anus to the cecum. Each colon was fixed flat between sheets of filter paper in 10% neutral buffered formalin for 24 h. All colons were stained with 0.1% methylene blue (Sigma-Aldrich, St. Louis, MO, USA) in saline and scored under a light microscope for the number of ACF per colon according to the procedure of Bird (22).
AOM/DSS-induced colon carcinogenesis experiment
DSS (MW: 36,000–50,000) was purchased from Sigma-Aldrich. Male mice, including C57BL/6 wild-type and SphK1 KO (C57BL/6 background) mice, were treated with AOM or saline (10 mg/kg, i.p.) at 6 wk of age and, 1 wk later, with 1% DSS in the drinking water for 1 wk. Then, all animals were given tap water until the end of the experiment. All animals were sacrificed by CO2 suffocation followed by cervical dislocation at 27 wk of age. All animals received BrdU (Sigma, 50 mg/kg, i.p.) 1 h before sacrifice. Blood samples were collected via heart puncture. After laparotomy, the entire intestines were resected and opened longitudinally, and the contents were flushed with normal saline. All colon tumors (Fig. 3A) were carefully examined for number and size. The length (L), width (W), and depth (D) of each tumor were measured with calipers, and tumor volume (V) was calculated using the formula V = L · W · D · π/6. Colon tumors and normal tissues were fixed in 10% buffered formalin and embedded in paraffin blocks for histological evaluation. Diagnosis of intestinal tumors using hematoxylin- and eosin-stained sections was performed according to the classification of Krutovskikh (23). The remaining colon tumors and segments of normal mucosa were snap-frozen in liquid nitrogen and stored at −80°C for subsequent analyses of the sphingolipid profile and RNA extraction for real-time reverse transcriptase-PCR (RT-PCR). Serial sections of colon tumor and normal mucosa were used for immunohistochemical staining.
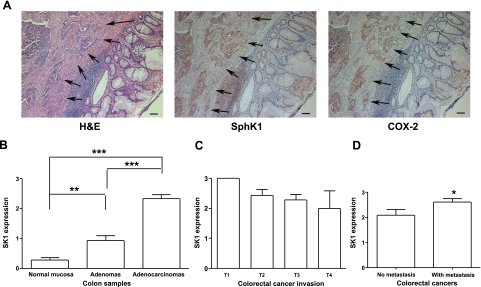
Figure 1.
SphK1 expression in human colon tissues. A) Representative immunohistochemical photos of human colorectal cancer (arrows) with normal mucosa (H&E, SphK1, and COX-2). Scale bars = 100 μm. B) Summary of SphK1 expression levels in normal mucosa (n=33), adenomas (n=14) and adenocarcinomas (n=33). **P < 0.01 vs. normal mucosa, ***P < 0.001 vs. normal mucosa and adenomas. C) SphK1 expression in invasiveness of primary colorectal cancers: T1 (n=2), tumor invades submucosa; T2 (n=7), tumor invades muscularis propria; T3 (n=21), tumor invades through muscularis propria into subserosa or into nonperitonealized pericolic or perirectal tissues; T4 (n=3), tumor directly invades other organs or structures and/or perforates visceral peritoneum. D) SphK1 expression levels in primary colorectal cancers with metastasis (n=21) and those without metastasis (n=12, *P<0.05). Error bars = se.
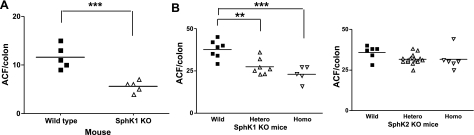
Figure 2.
Effects of SphK deficiency on AOM-induced ACF formation. A) ACF formation in SphK1 KO mice with C57BL/6 background. B) ACF formation in SphK1 and SphK2 KO mice with Balb/c background, including wild-type (WT), heterozygous, and homozygous mice. Bars represent group median; symbols represent ACF number of each animal. **P < 0.01, ***P < 0.001 vs. WT mice.
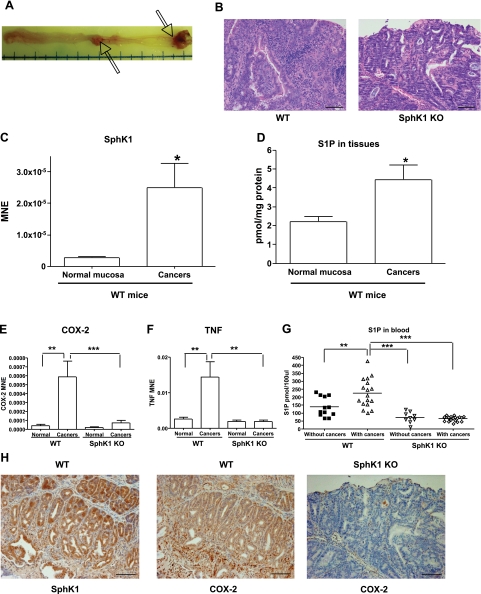
Figure 3.
AOM/DSS mouse colon carcinogenesis model. A) Macroscopic findings of colon tumors (arrows) induced by AOM/DSS in wild-type mouse. B) Representative photos of H&E staining of colon cancers collected from WT and SphK1 KO mice treated with AOM/DSS. C) Real-time RT-PCR results of SphK1 expression in normal mucosa and colon cancer tissues in WT mice (n=5). D) S1P measurement in normal mucosa and colon cancer tissues in WT mice (n=5). E, F) Results of real-time RT-PCR in normal mucosa and colon cancer tissues collected from WT and SphK1 KO mice for COX-2 (E) and TNF (F) (n=5). G) S1P measurement in blood samples collected from WT and SphK1 KO mice with or without colon cancers. The bars represent the median of each group; each symbol represents level of S1P in blood of each animal. H) Immunohistochemistry of colon cancer tissues for SphK1 and COX-2 in WT mice, and COX-2 in SphK1 KO mouse. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars = 50 μm. Error bars = se.
Levels of S1P in colon tissues and blood samples
Sphingolipids in colon tissues and blood samples were analyzed by sphingolipid profiling using tandem mass spectrometry (LS/MS) with positive mode electrospray ionization (EMS) in the Lipidomics Core Facility, MUSC, as described previously (13). Results were expressed as picomoles sphingolipids per milligram protein for colon tissues and picomoles sphingolipids per 100 μl for blood samples.
Real-time RT-PCR
RNA was extracted from colon tissues including cancers and paired normal mucosa of mice treated with AOM/DSS using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA) per the manufacturer’s instructions. cDNA was synthesized from total RNA (1 μg) using Moloney murine leukemia virus (M-MLV) RT (Promega, Madison, WI, USA) and oligo d (T)12-16. Real-time RT-PCR was performed on an iCycler iQ Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) with iQ SYBRE Green Supermix (Bio-Rad). The following primers were used for real-time PCR amplification: mouse SphK1, forward primer 5′-ACAGTGGGCACCTTCTTTC-3′ and reverse primer 5′-CTTCTGCACCAGTGTAGAGGC-3′; COX-2, forward primer 5′-AAGCGAGGACCTGGGTTCA-3′ and reverse primer 5′-AAGGCGCAGTTTATGTTGTCTGT-3′; TNF, forward primer 5′-AATGGCCTCCCTCTCATCAGTT-3′ and reverse primer 5′-CCACTTGGTGGTTTGCTACGA-3′; and β-actin, forward primer 5′-TCCTCCCTGGAGAAGAGCTA-3′ and reverse primer 5′-CCAGACAGCACTGTGTTGGC-3′. The real-time PCR conditions were as follows: initial step at 95°C for 3 min and cycles (n=40) consisted of 10 s at 95°C, followed by 1 min annealing/extension at 55°C. All reactions were performed in triplicate. Data were analyzed with Q-Gene software (24) and expressed as mean normalized expression (MNE). MNE is directly proportional to the amount of RNA of the target gene (SphK1, COX-2, or TNF) relative to the amount of RNA of the reference gene, β-actin.
Cell proliferation and apoptosis analyses
Cell proliferation was analyzed by BrdU incorporation in cancer cells in wild-type and SphK1 KO mice using immunohistochemistry. Apoptosis was analyzed by cleaved caspase-3 immunohistochemistry in cancer tissues in wild type and SphK1 KO mice. Immunohistochemically BrdU-positive cells were considered to be S-phase cells, and immunohistochemically caspase-3-positive cells were considered to be apoptotic. All cancer cells were counted in each colon cancer. For analyses of BrdU and apoptosis, only well-defined and darkly stained cells were counted using a microscope. The percentage of labeled cells (labeling index) was determined by calculating the labeled cell number: total cell number × 100.
Statistical analysis
Human SphK1 expression measurements at different stages of colon tumor development were analyzed by one-way ANOVA and Bonferroni’s multiple comparison tests among groups. ACF numbers were analyzed with Student’s t test between SphK1 KO and wild-type mice. In Balb/c background mice, ACF numbers were analyzed by one-way ANOVA and Bonferroni’s multiple comparison tests among homozygous, heterozygous, and wild-type mice. Levels of SphK1 mRNA and S1P in colon tissues of wild-type mice were analyzed with the unpaired Student’s t test between normal mucosa and cancers. mRNA expression of COX-2 and TNF in colon tissues were analyzed by one-way ANOVA and Bonferroni’s multiple comparison tests among groups. Levels of S1P in blood were analyzed by one-way ANOVA and Bonferroni’s multiple comparison tests among groups. In the AOM/DSS colon carcinogenesis model, tumor incidence, expressed as the percentage of tumor-bearing animals, was analyzed with the χ2 probability test, whereas tumor multiplicity, expressed as the mean number of tumors per mouse, and tumor volume (mm3) were analyzed with the unpaired Student’s t test between SphK1 KO and wild-type mice. BrdU and apoptotic indices were analyzed with the unpaired Student’s t test between SphK1 KO and wild-type mice. Differences were considered statistically significant at P < 0.05.
RESULTS
SphK1 expression in human colon samples
We first determined whether SphK1 is expressed in human colon tumor samples using immunohistochemistry performed with human SphK1 antibody (19). Human colorectal surgical samples, including 7 normal colon mucosa, 26 normal colon mucosa from cancer patients, 14 adenomas, 33 primary sites of adenocarcinomas, and 14 metastatic lesions of adenocarcinomas, including 7 lymph node and 7 liver metastases, were examined. The results are summarized in Table 1. SphK1 was expressed to a very limited extent in normal epithelial mucosa, which is in agreement with our previous findings (13) and data from others (25). Similarly, SphK1 expression was detected at low levels in the normal epithelium adjacent to colon adenocarcinomas (Fig. 1A, arrows indicate cancer tissues). In adenomas, SphK1 expression varied from negative to moderately positive. No differences were observed in SphK1 expression with respect to tumor size (>2 vs. ≤2 cm) or type of adenomas including tubular, tubulovillous, and villous adenomas (data not shown). SphK1 expression in adenomas, however, was significantly higher than in normal mucosa when the score of SphK1 expression was calculated (Fig. 1B, P<0.01). On the other hand, almost all adenocarcinoma samples (89%, 42/47) showed positive SphK1 immunostaining. Moreover, 100% of metastatic lesions of adenocarcinomas expressed high levels of SphK1. SphK1 expression in adenocarcinomas was significantly higher than in both normal mucosa and adenomas (Fig. 1B, P<0.001). These results clearly suggest that SphK1 expression may be related to colon tumor progression from normal mucosa to adenomas and then to adenocarcinomas. However, tumor invasiveness (depth of tumor at site) did not appear to be related to SphK1 expression (Fig. 1C).
TABLE 1.
Summary of immunohistochemistry for SphK1 and COX-2 in human colorectal tissues
| Tissue
|
n
|
SphK1 staining
|
COX-2 staining
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| – | ± | + | 2+ | – | ± | + | 2+ | ||
| Normal mucosa | 33 | 24 (73) | 9 (27) | 27 (82) | 6 (18) | ||||
| Adenoma | 14 | 3 (21) | 9 (64) | 2 (14) | 8 (57) | 4 (29) | 2 (14) | ||
| Adenocarcinoma | 33 | 5 (15) | 12 (36) | 16 (48) | 3 (9) | 13 (39) | 13 (39) | 4 (12) | |
| Metastasis | 14 | 6 (43) | 8 (57) | 6 (43) | 5 (36) | 3 (21) | |||
Values in parentheses represent percentages. −, no staining; ±, weak staining in <50% of cells; +, moderate staining in >50% of cells; 2+, strong staining in >50% of cells.
Because high SphK1 expression was observed in all metastatic lesions of colon cancers, we next investigated whether SphK1 expression is associated with metastatic potential. SphK1 expression was analyzed in 33 primary colon cancers, including 14 cancers with metastases and 19 cancers without metastases. Interestingly, SphK1 expression in metastatic primary colon cancer tissues was significantly higher than that of nonmetastatic primary cancers (Fig. 1D, P<0.05). The results suggest that higher expression of SphK1 in cancers may enhance the metastatic potential.
Next, COX-2 expression was measured in the same tumor and metastatic samples (Table 1). COX-2 staining was noted in only 52% (17/33) of adenocarcinomas and 57% (8/14) of metastatic lesions. COX-2 was also expressed in a limited fashion in normal mucosa. In adenomas, the same 2 samples showed positive staining with SphK1 and COX-2. Interestingly, all COX-2-positive colon tumors were positive for SphK1 expression, but not all SphK1-positive tumors were COX-2-positive. These results are consistent with previous data observed in AOM-induced rat colon cancers (13).
SphK1 deficiency inhibits ACF formation induced by AOM
To determine whether SphK 1 is required for early progression of colon carcinogenesis, the effects of SphK1 deficiency in early stages of AOM-induced colon carcinogenesis were next examined. Results of ACF numbers in wild-type and SphK1 KO mice are summarized in Fig. 2A. ACF formation was detected in the colons of all mice treated with AOM, but not in the colons of mice treated with vehicle control (data not shown). The number of ACF per colon was significantly reduced in SphK1 homozygous KO mice compared to wild-type mice by 51.7% (mean numbers of ACF/colon were 11.6 and 5.6 in wild-type and SphK1 KO mice, respectively; P<0.001; Fig. 2A). To confirm the data and examine effects of SphK2 in early stages of colon carcinogenesis, we next analyzed ACF formation in SphK1 KO and SphK2 KO mice with a different background (Balb/c). The results are summarized in Fig. 2B. As expected, the numbers of ACF per colon were significantly reduced in SphK1 homozygous and heterozygous KO mice compared to that in wild-type mice by 27% (P<0.01) and 39% (P<0.001), respectively (mean numbers of ACF/colon were 37.7 in wild-type, 27.4 in heterozygous, and 23.0 in homozygous SphK1 KO mice). In contrast, when compared to wild-type mice, heterozygous or homozygous SphK2 KO mice showed no differences in AOM-induced ACF formation. No weight loss or other abnormal signs were observed in the treated animals. These results indicate that SphK1, but not SphK2, may have a role in the early stages of colon carcinogenesis.
SphK1 expression and S1P production are increased in AOM/DSS-induced colon cancers
To examine the role of the SphK1/S1P pathway in inflammation-induced colon carcinogenesis, we utilized an AOM/DSS inflammation-related colon carcinogenesis model in wild-type and SphK1 KO mice (18). Representative macroscopic and microscopic figures of colon tumors are shown in Fig. 3A, B, respectively. First, SphK1 expression and S1P production in colon cancer tissues in wild-type mice treated with AOM/DSS were analyzed by real-time RT-PCR (Fig. 3C) and tandem LS/MS spectrometry (Fig. 3D), respectively. Levels of SphK1 mRNA and S1P were significantly elevated in colon cancer tissues compared to normal mucosa in wild-type mice (P<0.05). The results were confirmed by immunohistochemistry for SphK1 showing positive staining in colon cancer tissues induced by AOM/DSS in wild-type mice (Fig. 3H). Moreover, the levels of S1P in blood collected from mice bearing colon cancers were significantly higher than those from mice without colon cancers in wild-type mice (Fig. 3G, P<0.01). These results demonstrate that the SphK1/S1P pathway is activated in inflammation-induced colon carcinogenesis.
SphK1 deficiency reduces inflammation-induced colon carcinogenesis
Next, we investigated whether SphK1 is necessary for colon carcinogenesis, using an AOM/DSS inflammation-related colon carcinogenesis SphK1 KO mouse model. All animals were sacrificed at 20 wk after AOM injection. At the end of the experimental phase, 29 SphK1 KO and 30 wild-type mice were alive, and no abnormal changes (intestinal tumors) were observed in wild-type and SphK1 KO mice treated with saline. All colon tumors were diagnosed by routine hematoxylin and eosin (H&E) staining with pathological evaluation. The final results of colon tumors are summarized in Table 2. It was found that 22 wild-type mice developed colon tumors (73%, 22/30), including adenomas in 7 mice (23%, 7/30) and adenocarcinomas in 18 mice (60%, 18/30). In contrast, only 11 SphK1 KO mice developed colon tumors (38%, 11/29, P<0.01 vs. wild-type mice) including adenomas in 3 mice (10%, 3/29) and adenocarcinomas in 9 mice (31%, 9/29, P<0.01 vs. wild-type mice). In wild-type mice, 1.17 tumors per mouse were found while only 0.41 tumors per mouse were found in SphK1 KO mice (P<0.005, 65% inhibition in SphK1 KO mice). We found only 0.31 adenocarcinomas per mouse in SphK1 KO mice and 0.97 adenocarcinomas per mouse in wild-type mice (P<0.01, 68% inhibition in SphK1 KO mice). These results indicate that SphK1 deficiency significantly inhibited the development of colon tumors induced by AOM/DSS. SphK1 deficiency appeared to reduce colon cancer volume, but this reduction did not reach statistical significance. These results suggest that SphK1 plays a pivotal role in inflammation-induced colon carcinogenesis.
TABLE 2.
Results of AOM/DSS-induced colon carcinogenesis in SK1 KO mice
| Mouse
|
Incidence (% with tumors)
|
Multiplicity (tumors/mouse)
|
Cancer volume (mm3)
|
||||
|---|---|---|---|---|---|---|---|
| Total | Adenoma | ADC | Total | Adenoma | ADC | ||
| WT | 73 | 23 | 60 | 1.17 ± 0.20 | 0.23 ± 0.08 | 0.97 ± 0.21 | 17.4 ± 4.41 |
| SK1 KO | 38 (48)** | 10 (57) | 31 (48)** | 0.41 ± 0.11# | 0.10 ± 0.06 | 0.31 ± 0.09## | 4.77 ± 1.86 |
Totals include adenomas and adenocarcinomas (ADC). Values in parentheses represent percentage inhibition from wild-type mice. Multiplicity and volume values are means ± se.
P< 0.01 vs. wild type; χ2 test.
P< 0.005,
P< 0.01 vs. wild type; Student’s ttest.
Colon cancers induced by AOM/DSS were found to have more inflammation in wild-type mice than SphK1 KO mice (Fig. 3B). COX-2 and TNF expression levels were significantly elevated in colon cancer tissues compared to normal mucosa in wild-type mice (Fig. 3E, F, respectively; P<0.01). Immunohistochemical analyses showed that SphK1 and COX-2 protein levels were elevated in colon cancer tissues in wild-type mice treated with AOM/DSS (Fig. 3H). Interestingly, very low COX-2 expression was observed in colon cancers in SphK1 KO mice. These results were confirmed by real-time RT-PCR. mRNA of COX-2 and TNF extracted from colon cancers in SphK1 KO mice were significantly decreased compared to those in wild-type mice (Fig. 3E, F; P<0.001 and P<0.01, respectively). These results lend support to the fact that there is induction of the TNF/SphK1/COX-2 axis in colon carcinogenesis and provide evidence that SphK1 may function upstream of the COX-2 pathway in vivo. We also analyzed S1P levels in blood collected from wild-type and SphK1 KO mice treated with AOM/DSS using tandem LS/MS spectrometry (Fig. 3G). As expected, levels of S1P in blood samples collected from mice bearing colon cancers were significantly decreased in SphK1 KO mice compared to wild-type mice (P<0.001). In mice without colon cancers, S1P levels in blood were lower in SphK1 KO mice than those in wild-type mice. Moreover, it is noteworthy that S1P levels were not different between SphK1 KO mice with colon cancers and SphK1 KO mice without colon cancers, indicating that SphK2 is unlikely to have a role in this colon carcinogenesis model.
Inhibitory mechanism of SphK1 deficiency in colon carcinogenesis
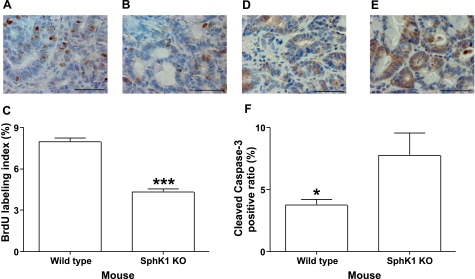
To investigate the mechanism by which SphK1 deficiency inhibits colon carcinogenesis, a BrdU incorporation assay was conducted to quantify cell proliferation using immunohistochemistry. As shown in Fig. 4A, B, BrdU-positive nuclei were observed in cancer cells of wild-type mice (Fig. 4A). In contrast, BrdU-positive nuclei were significantly reduced in cancer cells in SphK1 KO mice (Fig. 4B). The results of a BrdU labeling index in colon cancers are summarized in Fig. 4C. These results suggest that SphK1 deficiency significantly decreased cell proliferation in colon cancers (4.32 vs. 7.97 in SphK1 KO and wild-type mice, respectively; P<0.001). We also determined the effects of SphK1 deficiency on apoptosis in colon cancers. Cleaved caspase-3 was evaluated by immunohistochemical analysis, and it was detected in 3.78% of colon cancer cells of wild-type mice (Fig. 4D, F). In contrast, SphK1 KO mice had more than 7.77% apoptotic colon cancer cells (Figs. 4E, F). Thus, SphK1 deficiency significantly increased apoptotic cancerous cells (P<0.05). Collectively, the results suggest that SphK1 may control cell growth and survival in colon cancers.
Figure 4.
Effects of SphK1 deficiency on cell proliferation and apoptosis in colon cancers induced by AOM/DSS. A, B) Representative photos of BrdU immunohistochemistry in colon cancer from WT (A) and SphK1 KO (B) mice. C) Results of cell proliferation in colon cancer from wild type and SphK1 KO mice (n=5). D, E) Representative photos of cleaved caspase-3 immunohistochemistry in colon cancers from WT (D) and SphK1 KO (E) mice. F) Results of apoptotic index (cleaved caspase-3 positive ratio) in colon cancers from WT and SphK1 KO mice (n=5). *P < 0.05; ***P < 0.001. Scale bars = 50 μm. Error bars = se.
DISCUSSION
The results from this study provide strong evidence of a role for the SphK1/S1P pathway in colon carcinogenesis. The results show that SphK1 is overexpressed in human colon tumors, including adenomas and adenocarcinomas, and that SphK1 expression is higher in primary colon cancers with metastases than in those without metastases. Also, SphK1 mRNA and S1P are upregulated in colon cancer tissues in mice: S1P in blood collected from mice with colon cancer is higher than in mice without colon cancer. SphK1, but not SphK2, deficiency significantly inhibited AOM-induced ACF formation, a preneoplastic lesion of colon cancer. Finally, SphK1 deficiency significantly inhibited colon cancer development with reduction of cell proliferation and induction of apoptosis.
Interestingly, the results from this study suggest a role for SphK1 in the early stages of colon cancer development. Colonic ACF are thought to be preneoplastic lesions of colon cancer in a model of progressive malignant development. SphK1 KO mice significantly inhibited ACF formation and colon cancer incidence.
Recently, Kohno et al. (16) discussed the role of SphK1 and S1P in intestinal polyp formation, another preneoplastic lesion, in Min mice. In this model, SphK1 deficiency reduced only the size but not the incidence of polyps. The differences between these experiments and the current ones may be attributed to the unique models employed. The most important difference between AOM-induced ACF formation and intestinal polyp formation in Min mice is the site of tumor development. ACF develop in the colon, whereas polyps in the Min mouse mainly develop in the small intestines. SphK1 is reported to be the predominant SK in the colon, but in the small intestine, both SphK1 & SphK2 contribute to SphK activity (26). In the present study, we found that levels of S1P were not different in between SphK1 KO mice with and without colon tumors induced by AOM/DSS, indicating that SphK2 may not contribute to S1P production in this colon carcinogenesis model. The contribution of SphK1 vs. SphK2 to S1P levels in the Min mouse is unknown. However, work by Kohno et al. (16) suggests that S1P in intestinal polyps was not significantly different between wild-type and SphK1 KO mice with a Min background, indicating that SphK2 may contribute to S1P production in intestinal polyp formation in the Min mouse model. Thus, SphK1 may possibly play a greater role in colon carcinogenesis than in polyp formation in the small intestine. We also found that SphK1, but not SphK2, deficiency significantly inhibited AOM-induced ACF formation in different background (Balb/c) KO mice. Our data strongly support the hypothesis that SphK1, but not SphK2, plays a pivotal role in the early stages of colon carcinogenesis.
The results from this study also raise the possibility that SphK1 may have additional roles in later stages of colon cancer progression. SphK1 mRNA expression has been reported to be upregulated in human colon cancers compared to adjacent normal mucosa, as evaluated by Northern blot (27) and protein measurements via immunohistochemistry (16). The current immunohistochemistry results revealed that SphK1 is also overexpressed in adenomas. More important, the results from this study showed that SphK1 was significantly higher in adenocarcinomas than in adenomas in human colon samples, and that there was progressively higher expression of SphK1 in primary sites of colon cancers with metastases than in those without metastases. These results suggest that SphK1 may play roles in several steps of colon carcinogenesis. This hypothesis is supported by cell-based studies demonstrating antiapoptotic, promigratory, and proangiogenic functions for SphK1, properties that would enhance growth, survival, and metastasis of advanced tumors (6).
The results from this study advance the emerging paradigm on the role of inflammation and proinflammatory mediators in the pathogenesis of cancer, especially colon cancer. Chronic inflammation may promote colonic tumor formation and progression because IBD patients have an increased risk for colorectal cancer development. Mechanistically, the COX-2/PGE2 pathway has become one of the most studied and important inflammatory regulators for colon carcinogenesis. Accumulating evidence suggests that inhibition of COX-2 reduces colon carcinogenesis, whereas PGE2 enhances the process. Substantial and ongoing studies implicate the SphK1/S1P pathway in COX-2 regulation, especially in response to proinflammatory cytokines. In HT-29 colon cancer cells, the SphK1/S1P pathway was shown to mediate COX-2 expression and PGE2 production in response to TNF and IL1 (13). Current results show that SphK1 deficiency significantly inhibited AOM/DSS-induced COX-2 expression, providing an in vivo mechanistic link between SphK1 and COX-2. Functionally, SphK1 deficiency reduced colon carcinogenesis, indicating that SphK1 is involved in inflammation-related colon carcinogenesis. Taken together, these results suggest that the SphK1/S1P pathway may mediate COX-2 expression as a downstream target in inflammation-mediated colon cancer.
Mechanistically, the results from this study not only implicate SphK1 in the induction of COX-2 during colon carcinogenesis, but also suggest a role for this pathway and the lipid mediators it controls in regulating tumor growth and viability. Tumor size is associated with a balance between cell proliferation and apoptosis in tumor cells. SphK1 deficiency significantly reduced cell proliferation and enhanced apoptosis in colon tumors induced by AOM/DSS, compared to those in wild-type mice. The outcome of these 2 processes would serve to enhance tumor growth and survival.
The results raise the possibility that SphK1 may serve as a novel target for chemoprevention and chemotherapy of colon cancer. SphK1 acts upstream of COX-2 and therefore may have the same benefits of COX-2 inhibition in colon cancer, but perhaps without some of the adverse systemic and cardiovascular effects of COX-2 inhibition that have resulted in abandoning COX-2 inhibitors in the treatment and prevention of colon cancer. This possibility requires further study.
Of note, blood S1P levels in mice with colon cancers are significantly higher than those in mice without colon cancers, suggesting a possible biomarker for early detection of colon tumors. This possibility also requires further study.
In conclusion, these results show that SphK1 is upregulated in human colon tumors, including adenomas and adenocarcinomas, and SphK1 expression is higher in cancers with metastases, indicating that SphK1 expression may be associated with colon tumor progression and metastasis. We also found that SphK1, not SphK2, deficiency significantly inhibits colon ACF formation and cancer development, indicating that SphK1 may play a pivotal role in both early and late stages of colon carcinogenesis. Thus, the SphK1/S1P pathway may be a viable target for colon cancer chemoprevention/chemotherapy.
Acknowledgments
We thank Margaret Romano and the Tumor Bank for technical assistance and Dr. Takuji Tanaka (Kanazawa Medical University, Kanazawa, Japan) for his diagnostic review of colon tumors. Also, we thank the Lipidomics Core Facility and Animal Carcinogenesis Core, the Hollings Cancer Center, MUSC. This work was supported by NIH grants (P20RR17677 to T.K.; P01CA097132 to Y.A.H. and L.M.O.; R01GM62887 to L.M.O.) and a seed grant to T.K. from the Hollings Cancer Center, MUSC. This work was conducted in a facility constructed with support from the NIH Extramural Research Facilities Program of the National Center for Research Resources (C06RR015455).
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun M J. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun Y A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acids Res Mol Biol. 2002;71:493–511. doi: 10.1016/s0079-6603(02)71049-0. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 2000;476:55–57. doi: 10.1016/s0014-5793(00)01670-7. [DOI] [PubMed] [Google Scholar]
- Pyne S, Pyne N J. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Gamble J R, Wang L, Pitson S M, Moretti P A, Wattenberg B W, D'Andrea R J, Vadas M A. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Rao C V, Seibert K, Reddy B S. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- Nakatsugi S, Fukutake M, Takahashi M, Fukuda K, Isoi T, Taniguchi Y, Sugimura T, Wakabayashi K. Suppression of intestinal polyp development by nimesulide, a selective cyclooxygenase-2 inhibitor, in Min mice. Jpn J Cancer Res. 1997;88:1117–1120. doi: 10.1111/j.1349-7006.1997.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach G, Lynch P M, Phillips R K, Wallace M H, Hawk E, Gordon G B, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su L K, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- Hansen-Petrik M B, McEntee M F, Jull B, Shi H, Zemel M B, Whelan J. Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res. 2002;62:403–408. [PubMed] [Google Scholar]
- Pettus B J, Bielawski J, Porcelli A M, Reames D L, Johnson K R, Morrow J, Chalfant C E, Obeid L M, Hannun Y A. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Osta W, Johnson K R, Pettus B J, Bielawski J, Tanaka T, Wargovich M J, Reddy B S, Hannun Y A, Obeid L M, Zhou D. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pettus B J, Kitatani K, Chalfant C E, Taha T A, Kawamori T, Bielawski J, Obeid L M, Hannun Y A. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68:330–335. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- Kohno M, Momoi M, Oo M L, Paik J H, Lee Y M, Venkataraman K, Ai Y, Ristimaki A P, Fyrst H, Sano H, Rosenberg D, Saba J D, Proia R L, Hla T. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K R, Becker K P, Facchinetti M M, Hannun Y A, Obeid L M. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J Biol Chem. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- Allende M L, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane C A, Mandala S, Spiegel S, Proia R L. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- Bird R P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- Krutovskikh V A, Turusov V S. Tumours of the intestines. IARC Sci Publ. 1994:195–221. [PubMed] [Google Scholar]
- Muller P Y, Janovjak H, Miserez A R, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. BioTechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Murate T, Banno Y, K T K, Watanabe K, Mori N, Wada A, Igarashi Y, Takagi A, Kojima T, Asano H, Akao Y, Yoshida S, Saito H, Nozawa Y. Cell type-specific localization of sphingosine kinase 1a in human tissues. J Histochem Cytochem. 2001;49:845–855. doi: 10.1177/002215540104900705. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Kihara A, Igarashi Y. Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem Biophys Res Commun. 2003;309:155–160. doi: 10.1016/s0006-291x(03)01551-1. [DOI] [PubMed] [Google Scholar]
- French K J, Schrecengost R S, Lee B D, Zhuang Y, Smith S N, Eberly J L, Yun J K, Smith C D. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]