FIGURE 3.
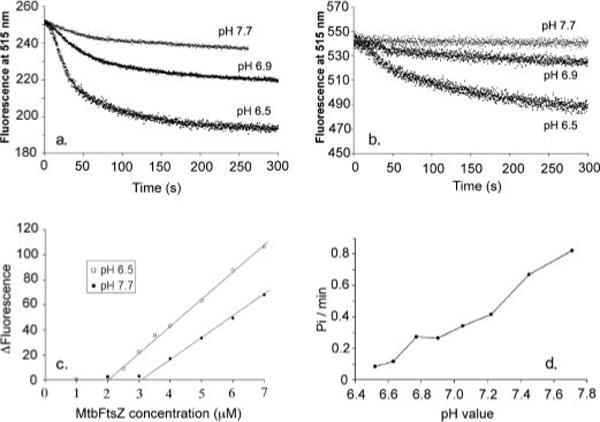
a, MtbFtsZ assembly kinetics measured by FRET with 3.5 μm fluorescein-labeled protein (donor) plus 3.5 μm tetramethylrhodamine-labeled protein (acceptor). Fluorescence at time zero was normalized to the same value, 250. The extent of fluorescence change was greater at lower pH. However, the kinetics of assembly were similar at each pH. b, the homo-FRET signal of fluorescein-labeled MtbFtsZ without acceptor. The protein concentration was 7 μm. c, the equilibrium FRET signal (the absolute change in donor fluorescence from 0 to 120 s) is plotted as a function of FtsZ concentration. The critical concentration is 2 μm in MMK buffer (50 mm MES, pH 6.5, 100 mm KAc, 5 mm MgAc, 1 mm EGTA) and 3.2 μm in HMK buffer (50 mm HEPES, pH 7.7, 100 mm KAc, 5 mm MgAc, 1 mm EGTA). d, MtbFtsZ has different GTPase activity at different pH. The GTPase activity is about 0.08 GTP/min/FtsZ at pH 6.5 and increases to about 0.8 at pH 7.7. The results show an approximately linear increase in GTPase with increasing pH. All measurements were done at room temperature.
