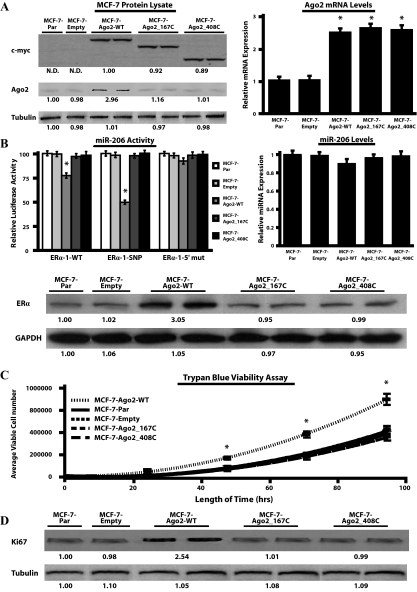
Figure 4.
Overexpression of Ago2 induces alterations in the phenotype of MCF-7 cells. A, MCF-7-Ago2 transfectants (MCF-7-Ago2-WT, MCF-7-Ago2_167C, and MCF-7-Ago2_408C) were screened for myc-epitope and Ago2 expression by Western blot analysis (left panel). The densitometry readings depicted below each Western blot represent the mean myc-epitope, Ago2, and tubulin expression. Real-time PCR was performed to monitor Ago2 transcript levels in the MCF-7 transfectants (right panel). Values were normalized to RPL-19 and reported as relative to MCF-7 parental (MCF-7-Par) cells. B, Luciferase assays using the pIS-ERα-1 constructs were performed to monitor miR-206 activity in the MCF-7-Ago2 transfectants (left panel), whereas real-time PCR was performed to determine mature miR-206 levels (right panel). Values from the luciferase and real-time PCR assays were normalized to Renilla luciferase or 5s RNA, respectively, and reported as relative to the levels in MCF-7-Par cells. Western blots were also performed to measure endogenous ERα expression in the various MCF-7 transfectants (bottom panel). C, The number of viable MCF-7 transfectants was determined by Trypan blue growth curves as described in Materials and Methods. D, Western blot analysis of Ki67 expression levels in the MCF-7 transfectants. Numbers below bands represent the average densitometric readings for Ki67 and tubulin expression relative to MCF-7-Par cells. For all assays three independent experiments were performed in quadruplicate, and values were reported as the mean + sem. Data generated from the Ago2 transfectants are representative of three stably selected pools of cells with equivalent WT or mutant Ago2 expression (*, P < 0.05, compared with MCF-7-Par control). GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; N.D., nondetectable densitometric reading; SNP, single nucleotide polymorphism.