Abstract
Acute insulin resistance occurs after injury, hemorrhage, infection, and critical illness. However, little is known about the development of this acute insulin-resistant state. In the current study, we found that insulin resistance develops rapidly in skeletal muscle, with the earliest insulin signaling defects at 60 min. However, defects in insulin signaling were measurable even earlier in liver, by as soon as 15 min after hemorrhage. To begin to understand the mechanisms for the development of acute insulin resistance, serine phosphorylation of insulin receptor substrate (IRS)-1 and c-Jun N-terminal kinase phosphorylation/activation was investigated. These markers (and possible contributors) of insulin resistance were increased in the liver after hemorrhage but not measurable in skeletal muscle. Because glucocorticoids are important counterregulatory hormones responsible for glucose homeostasis, a glucocorticoid synthesis inhibitor, metyrapone, and a glucocorticoid receptor antagonist, RU486, were administered to adult rats prior to hemorrhage. In the liver, the defects of insulin signaling after hemorrhage, including reduced tyrosine phosphorylation of the insulin receptor and IRS-1, association between IRS-1 and phosphatidylinositol 3-kinase and serine phosphorylation of Akt in response to insulin were not altered by pretreatment of rats with metyrapone or RU486. In contrast, hemorrhage-induced defects in insulin signaling were dramatically reversed in skeletal muscle, indicating a prevention of insulin resistance in muscle. These results suggest that distinct mechanisms for hemorrhage-induced acute insulin resistance are present in these two tissues and that glucocorticoids are involved in the rapid development of insulin resistance in skeletal muscle, but not in the liver, after hemorrhage.
Glucocorticoids play a major role in the development of acute insulin resistance following hemorrhage in skeletal muscle, but not in the liver.
Insulin resistance, a decreased ability to respond to insulin, is associated with several chronic diseases including type 2 diabetes, hypertension, cardiovascular disease, polycystic ovary syndrome, and metabolic syndrome (1,2). A distinct and acutely developing form of insulin resistance is observed after injury, infection, and critical illness (3,4), in which hyperglycemia and hyperinsulinemia have been linked to increased mortality and morbidity (5,6). Intensive insulin therapy in critically ill patients is highly beneficial, resulting in a 34–50% reduction in mortality (7,8). The underlying cause for the hyperglycemia is the development of an acute form of peripheral insulin resistance. Insulin action starts by binding to its specific insulin receptor (IR), activation of the IR tyrosine kinase activity, and the subsequent phosphorylation of insulin receptor substrates (IRS), resulting in the activation of intracellular signaling cascades (9,10). One of the main pathways stimulated by insulin is the IRS/phosphatidylinositol 3-kinase (PI3K)/Akt pathway.
We now know that hepatic insulin resistance develops after experimental injury (11,12,13), but it is not known whether defects of insulin signaling occur in skeletal muscle after hemorrhage. Also, molecular mechanisms of acute insulin resistance may differ in different insulin target tissues (14). An understanding of tissue-specific insulin resistance, and each tissue’s contribution to the altered metabolism in response to injury, will likely promote development of new therapeutic interventions.
Possible causative factors of the acute insulin resistance are the counterregulatory hormones, in particular glucocorticoids (15,16). Glucocorticoid levels increase after various forms of trauma (17,18,19) and are often elevated in insulin-resistant patients (20), and cortisol levels can be reduced in critically ill patients by intensive insulin therapy (21). Glucocorticoids directly influence insulin sensitivity by interfering with components of the insulin signaling cascade (22,23). For instance, treatment of rats with glucocorticoids reduces IR protein, PI3K activity, and Akt activation in response to insulin (22,23).
In the present study, we examined the mechanisms that underlie the development of acute insulin resistance in liver and skeletal muscle and assess the role of glucocorticoids on insulin signaling after experimental injury. Our results indicate that the insulin resistance in skeletal muscle, but not in liver, is glucocorticoid dependent.
Materials and Methods
Animal model of surgical trauma and hemorrhage
Experiments were performed in accordance with the guidelines of the Care and Use of Laboratory Animals and the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Male Sprague Dawley rats (260–300 g; Harlan, Indianapolis, IN) were fasted 18–20 h before the surgery but were allowed water ad libitum. A model of trauma and hemorrhage in the rats, as previously described (11,12,13), was used in the present study. Briefly, rats were anesthetized with isoflurane inhalation (Mallinckrodt Veterinary, Mundelein, IL), a 5-cm ventral midline laparotomy was performed representing soft tissue trauma, and the abdomen was closed. Polyethylene-5 catheters (Becton Dickinson Intramedic, Sparks, MD) were placed in the right and left femoral arteries for bleeding and monitoring of mean arterial pressure. Rats in trauma and hemorrhage (TH) groups were bled rapidly to a mean arterial pressure of 35–40 mm Hg within 10 min. Once mean arterial pressure reached 40 mm Hg, timing of the hemorrhage period began and was maintained for the indicated times. Rats in trauma only (T) groups underwent the same laparotomy and catheterization but were not hemorrhaged.
Study design
Due to the considerable stress incurred by anesthesia and opening of the abdominal cavity to perform the insulin or saline injections (see below), it was impossible to have a completely untreated control group. Thus, trauma-alone rats (T0′) that were subjected to anesthesia, laparotomy, and catheterization, and then immediately killed, were selected as the baseline animals in the present study (11,12,13). Other trauma-only groups were subjected to the same procedures and then killed at 15 (T15′), 30 (T30′), 60 (T60′), or 90 (T90′) min after catheterization. Matched to these groups were the TH groups that were subjected to the same procedures as the T groups, but also subjected to hemorrhage, and then killed at the indicated time points.
Drugs and drug administration
Metyrapone and RU486 were purchased from Sigma (St. Louis, MO) and were dissolved in propylene glycol. For metyrapone-pretreated rats, animals received one injection of either metyrapone (150 mg/kg, sc) or vehicle (propylene glycol) 3 h before the surgery, a time and dose known to inhibit the stress-induced rise in plasma corticosterone level (24,25). For the RU486 experiments, rats received an injection of RU486 (25 mg/kg, sc) 1 h before surgery, a time and dose known to block glucocorticoid action (26,27).
Tissue and blood harvesting procedures
At the end points for each group (0′, 15′, 30′, 60′, and 90′), the abdominal cavity was opened, the portal vein exposed, and insulin (5 U) or saline injected into the portal vein. One minute after the injection, liver and triceps were removed and quickly frozen in liquid nitrogen for future analysis. Just before insulin or saline injection, blood was withdrawn from the inferior vena cava and centrifuged at 5000 × g for 15 min. The plasma was stored at −80 C.
Measurement of free fatty acids (FFAs) and corticosterone levels
FFA levels were measured by using a nonesterified fatty acid-HR assay (Wako Diagnostics, Richmond, VA) and corticosterone levels were determined via a RIA kit (MP Biomedicals, Orangeburg, NY).
Tissue extraction, immunoprecipitation, and immunoblotting analysis
Liver or muscle tissue (0.1 g) was homogenized in extraction buffer, as described previously and stored at −80 C until use (11,12,13,28). Protein concentrations were determined using the BCA method (Pierce, Rockford, IL).
For immunoblotting, protein was separated by SDS-PAGE (30 μg/lane) and analyzed by specific antibodies as indicated in the figures. Western blot results are typically representative of at least three independent experiments with similar results. Antibodies include anti-p85 subunit of PI3K (Upstate Biotechnology, Lake Placid, NY); antiphosphoserine (PS)-473-Akt and total Akt (Cell Signaling, Danvers, MA); antiphosphotyrosine (PY)-612-IRS-1, PS312-IRS-1, PY972-IR, phosphorylated c-Jun N-terminal kinase (JNK) 1/2 (Invitrogen, Carlsbad, CA); and total IRS-1, total IR, and total JNK (Santa Cruz Biotechnology, Santa Cruz, CA). The Precision Plus protein kaleidoscope standards were used to indicate the molecular weight of loading proteins (Bio-Rad Laboratories. Inc., Hercules, CA).
For immunoprecipitation, 1 mg protein from either liver or triceps was incubated with an antibody against IRS-1 overnight at 4 C. Protein G-agarose (Pharmacia Biotech, Providence, RI) was then added and incubated for another 4 h at 4 C. Immunoprecipitated proteins were immunoblotted with anti-IRS-1 and anti-p85 subunit of PI3K antibodies.
Densitometric and statistical analysis
Enhanced chemiluminescence images of immunoblots were scanned and quantified using Zero D-Scan (Scanalytics Corp., Fairfax, VA). All data were analyzed by one-way ANOVA using the InStat statistical program (GraphPad Software, Inc., San Diego, CA).
Results
Decreased insulin signaling in liver and skeletal muscle after trauma and hemorrhage
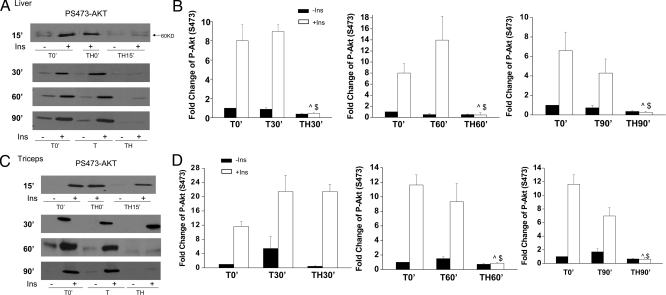
We previously reported that hepatic insulin signaling was almost completely lost at 90 min after trauma and hemorrhage (TH90′) compared with trauma-only (T0′, T90′) animals (11,12,13). Because hepatic insulin resistance might occur earlier than 90 min, several earlier time points were examined. The animals were hemorrhaged and blood pressure maintained at 35–40 mm Hg for 15 min (TH15′), 30 min (TH30′), and 60 min (TH60′), respectively. There was a profound loss of hepatic insulin-induced PS-Akt at all time points tested (Fig. 1A). There was no significant difference between the fold induction of PS-Akt in liver after insulin injection in the T (T0′, T30′, T60′, T90′) groups. However, there was a significant decrease of insulin’s ability to induce hepatic PS-Akt in all TH groups (Fig. 1B), indicative of a significant loss of insulin signaling.
Figure 1.
Decreased insulin signaling via phospho-Akt (P-Akt) at multiple time points after trauma and hemorrhage in the liver and triceps. Rats were subjected to T or TH. At 0′, 15′, 30′, 60′, and 90′ (with or without hemorrhage) either saline (−) or 5 U insulin (Ins; +) was injected via the portal vein. The liver and triceps were removed after 1 min. Tissue lysates were subjected to Western blotting with antibodies specific for PS473-Akt. Representative Western blots from TH0′, TH15′, TH30′, TH60′, and TH90′ in liver (A) and triceps (C) are presented. B and D, Autoradiographs from TH30′, TH60′, and TH90′ were quantified by scanning densitometry. The data are presented as mean ± sem fold change of PS473-Akt by insulin of three rats in each group. The phosphorylated protein levels in T0′ without insulin treatment were arbitrarily set to 1. ^, P < 0.01, compared with T-only group at the same time point with insulin injection; $, P < 0.01, compared with T0′ with insulin injection.
Because skeletal muscle is another important insulin target tissue and accounts for approximately 80% of insulin-induced glucose disposal (29), insulin signaling was also examined in skeletal muscle at the above time points to determine whether muscle also becomes insulin resistant. In contrast to hepatic insulin signaling, PS-Akt was still induced by insulin at both 15 min and 30 min after the combination of trauma and hemorrhage, although, as in liver, it was suppressed at 60 and 90 min after hemorrhage (Fig. 1, C and D). Thus, insulin resistance developed later in skeletal muscle than in liver after hemorrhage.
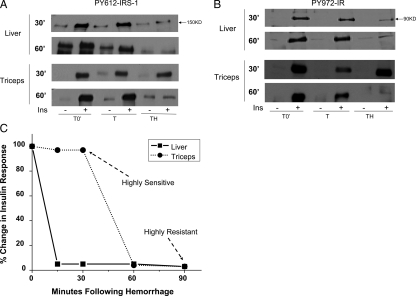
Experiments were performed to measure whether there were any changes in insulin-induced phosphorylation upstream of Akt at IRS-1 and the IR after TH. PY612 of IRS-1 was barely detectable in the liver after insulin stimulation at the TH30′ and TH60′ time points. In contrast, PY612-IRS-1 in triceps was induced by insulin at TH30′ and not significantly different from that of the T0′ or T30′ groups. However, insulin-induced PY612-IRS-1 was lost in triceps after 60 min of hemorrhage (Fig. 2A). Similar differences in phosphorylation of IR at tyrosine 972 were observed with insulin-induced PY972-IR at 30 min after hemorrhage in muscle but not in liver (Fig. 2B).
Figure 2.
Decreased insulin-induced tyrosine phosphorylation of IRS-1 and IR in the liver and triceps after TH. At the same time points and treatments described in Fig. 1, liver and triceps were removed after either saline (−) or 5 U insulin injection. A and B, Representative Western blots from TH30′ and TH60′ are presented. C, Time-dependent decrease in insulin responses in the liver and triceps after hemorrhage. There were three rats in each group. Ins, Insulin.
Thus, when the ability to respond to exogenous insulin was plotted against time, it was clear that hepatic insulin resistance develops earlier than in skeletal muscle after trauma and hemorrhage (Fig. 2C). This implies that distinct molecular mechanisms may be present between these two major insulin-responsive tissues.
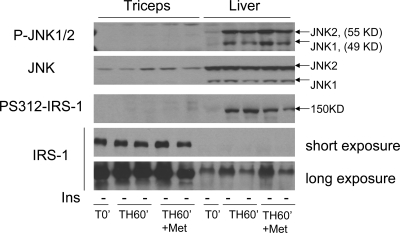
Increased serine phosphorylation of IRS-1 and phosphorylation of JNK1/2 in the liver and skeletal muscle after trauma and hemorrhage
TNFα increases after infection, injury, and chronic diseases and may contribute to insulin resistance (30). Our previous findings indicated an important role for TNFα in the acute development of hepatic insulin resistance after trauma and hemorrhage (12,13). However, whether it plays a role in skeletal muscle insulin resistance is unclear. TNFα can activate JNK signaling, and JNK can associate with, and phosphorylate, IRS-1 at serine residue 312 [the serine 312 position refers to the human protein, whereas the equivalent residue is serine 307 of the rat or mouse protein (31,32)], and this serine phosphorylation inhibits the ability of IRS-1 to act as a docking protein (30,33). Similar to our previous data (13), JNK1 and JNK2 were activated/phosphorylated in the liver, as detected by a phospho-specific JNK antibody. PS312-IRS-1 was also detected after hemorrhage in the liver (Fig. 3). In contrast, only a slight increase in JNK1 and JNK2 phosphorylation and PS312-IRS-1 was detected in skeletal muscle, even when the autoradiographs were greatly overexposed. There was a significant amount of total JNK protein in skeletal muscle, although less than in liver. Conversely, IRS-1 levels were higher in skeletal muscle than in liver (Fig. 3).
Figure 3.
Comparison of PS IRS-1 and JNK activation in the liver and triceps. At T0′, TH60′, and TH60′ with metyrapone treatment, the liver and triceps were removed and subjected to Western blot analysis. The blots were probed with specific anti-PS312-IRS-1, total IRS-1, anti-P-JNK1/2, and total JNK antibodies. Representative Western blots are presented (T0′-ins and TH60′-ins, three rats each; TH60′-ins+Met, five rats;). Ins, Insulin; Met, metyrapone.
FFAs and corticosterone levels after trauma and hemorrhage
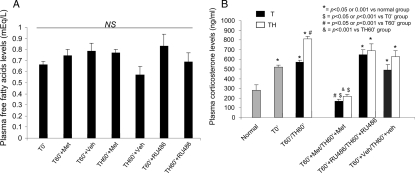
Elevated FFA levels can contribute to chronic forms of insulin resistance (34). To determine whether they play a role in the onset of acute insulin resistance after hemorrhage, the levels of FFAs were measured and their levels did not change after either T alone or TH (Fig. 4A, left bars 1, 3, and 5).
Figure 4.
Plasma FFA (A) and corticosterone (B) levels in differently treated rats. Metyrapone, RU486, or vehicle was administered as described in Materials and Methods. The rats were killed at the end of surgery at which time blood was collected for the determination of plasma FFA and corticosterone levels. Results are expressed as milliequivalents per liter plasma and nanograms corticosterone per milliliter plasma, respectively. Each value represents the mean ± se of six to 10 rats per group (T0′, six rats; normal, six rats; T60′, six rats; TH60′, six rats; T60′+Met, six rats; TH60′+Met, 10 rats; T60′+veh, six rats; TH60′+veh, six rats; TH60′+RU486, nine rats). Met, Metyrapone; Veh, vehicle; NS, not significant.
It was then asked whether glucocorticoids (corticosterone is the main plasma corticosteroid in rodents) was increased by the experimental procedures. There was an increase in corticosterone due to the anesthesia and surgeries (T0′) compared with normal animals. Little additional increase by 60 min in T (T60′) animals was observed (Fig. 4B, bars 1–3). There was an additional significant increase in circulating concentrations of corticosterone after the combination of TH (TH60′; Fig. 4B, bars 4 vs. 3). We also measured cortocosterone levels at 30 min after trauma and hemorrhage and found that the corticosterone level (617.6 ± 54.7 ng/ml) was significantly increased compared with normal animals but not increased as much as after 60 min of TH. Thus, corticosterone levels increased rapidly after TH, leading us to question its role in the development of hepatic and skeletal muscle insulin resistance.
Suppression of corticosterone synthesis did not alter the impaired hepatic insulin signaling after hemorrhage
Increased glucocorticoids can result in decreased insulin action and whether glucocorticoids contribute to the acute development of insulin resistance after hemorrhage is unknown. Therefore, the hemorrhage-induced increase in corticosterone levels was inhibited by treating rats with metyrapone, an inhibitor of corticosterone synthesis (24,25), 3 h before surgery. As expected, after T or TH, the prior treatment with metyrapone reduced corticosterone levels compared with vehicle-treated animals and below that in normal animals (Fig. 4B). Conversely, treatment with metyrapone did not alter FFA levels (Fig. 4A).
Treatment with metyrapone did not change the basal or hemorrhage-induced levels of phosphorylated JNK1/2 or PS312-IRS-1 in skeletal muscle or liver (Fig. 3). This indicates that the increase in hepatic JNK signaling or inhibitory serine phosphorylation of IRS-1 is not regulated by the increase of corticosterone levels after hemorrhage.
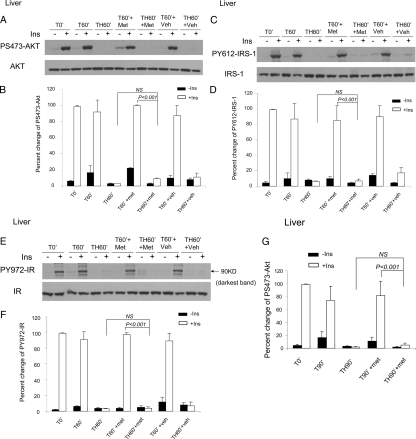
Suppression of corticosterone synthesis had little or no effect on the hemorrhage-induced reduction in insulin-stimulated PS-Akt in the liver (Fig. 5, A and B). Similarly, the reduction of hepatic insulin-induced tyrosine phosphorylation of IRS-1 and IR after hemorrhage were unaltered by pretreatment with metyrapone (Fig. 5, C–F). To confirm this observation, that metyrapone did not alter the insulin-resistant state in liver, insulin signaling was also measured after hemorrhage for 90 min (TH90′, Fig. 5G). These data, as at the TH60′ time point, indicate that blockade of the increased corticosterone levels that normally occur after hemorrhage did not alter the development of hepatic insulin resistance and the increase of P-JNK and PS-IRS-1 after hemorrhage (Fig. 3). We also studied the effect of metyrapone on tyrosine phosphorylation of IRS-2 in the liver; there was no measurable effect of metyrapone on insulin-induced tyrosine phosphorylation of IRS-2 (data were not shown).
Figure 5.
Insulin-induced phosphorylation of Akt, tyrosine phosphorylation of IRS-1, and IR autophosphorylation were not increased by metyrapone treatment in liver. Rats were subjected to T or TH. Metyrapone (150 mg/kg, sc) or vehicle was administered as described. At the end of surgery, either saline (−) or 5 U insulin was injected via the portal vein. Tissue lysates were subjected to Western blotting with antibodies specific for PS473-Akt or total Akt, PY-IRS-1 or total IRS-1, and PY-IR or total IR. Representative Western blots are presented in A, C, and E. B, D, F, and G, Autoradiographs were quantified by scanning densitometry and the data are presented as the mean ± se of three to five rats in each group (five rats in TH60′+ins+Met group, five rats in TH60′-ins+Met group, three rats in other groups). The phosphorylated protein levels in T0′ with insulin injection were arbitrarily set to 100%. A–F, T60′/TH60′; G, T90′/TH90′. Met, Metyrapone; Veh, vehicle; Ins, insulin; NS, not significant.
Suppression of corticosterone synthesis markedly reduced insulin resistance in skeletal muscle after trauma and hemorrhage
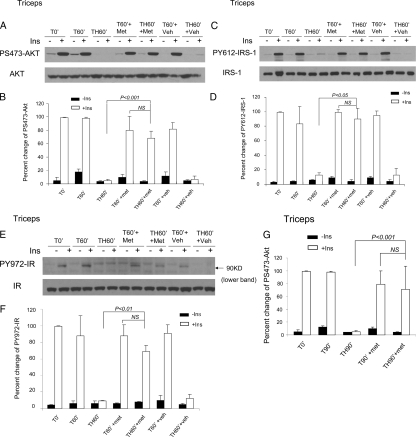
Although metyrapone did not rescue the hemorrhage-induced insulin resistance in liver, the action of glucocorticoids is tissue specific (16). The different time course of development of insulin resistance in liver vs. skeletal muscle (see Figs. 1 and 2) implies different molecular mechanisms for the hemorrhage-induced insulin resistance in these two tissues. When the phosphorylation of Akt was measured in skeletal muscle after hemorrhage and after pretreatment with metyrapone, there was a dramatic increase in the ability of insulin to stimulate Akt phosphorylation in skeletal muscle. Levels of insulin-induced PS-Akt, which were negligible after the combination of trauma and hemorrhage in triceps, were almost completely normalized by metyrapone pretreatment (Fig. 6, A and B).
Figure 6.
Effects of metyrapone on the impaired insulin signaling pathway in triceps. At the same time points and treatment described in Fig. 5, either saline (−) or 5 U insulin was injected into the portal vein, and 1 min later triceps was removed. Then 30 μg protein extracted from triceps were subjected to Western blot analysis by specific antibodies as described in Fig. 5. A, C, and E, Representative Western blots are presented. B, D, F, and G, Bars represent mean ± se from three to five rats in each group (five rats in TH60′+ins+Met group, five rats in TH60′-ins+Met group, three rats in other groups). A–F, T60′/TH60′; G, T90′/TH90′. Met, Metyrapone; Veh, vehicle; Ins, insulin; NS, not significant.
Upstream of Akt is IRS-1 and IR, so the ability of metyrapone pretreatment followed by trauma and hemorrhage on other members of the insulin signaling pathway was determined. Levels of insulin-induced PY612-IRS-1, which were negligible after the combination of trauma and hemorrhage in triceps, were almost completely normalized by metyrapone pretreatment (Fig. 6, C and D). Furthermore, the ability of insulin to induce PY972-IR in skeletal muscle was also restored by metyrapone pretreatment (Fig. 6, E and F). To confirm these observations, that metyrapone blocks the development of the insulin-resistant state in skeletal muscle, insulin signaling was measured to be near normal after hemorrhage for 90 min (TH90′, Fig. 6G).
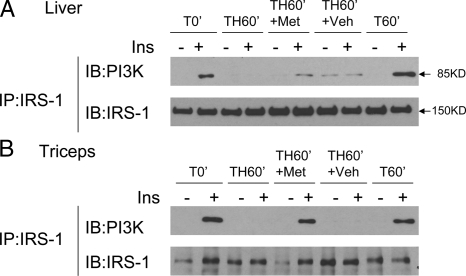
Insulin-induced phosphorylation of IRS-1 increases association of IRS-1 with PI3K, so the effects of metyrapone on insulin-stimulated IRS-1/PI3K association were also studied. In T animals (T0′, T60′), there was a strong association, as measured by coimmunoprecipitation, of IRS-1 and PI3K after insulin stimulation in the liver and skeletal muscle, and this association was lost after hemorrhage in the TH60′ animals (Fig. 7, A and B). After metyrapone pretreatment, there was little hepatic insulin-induced IRS-1/PI3K association in the TH60′ group in liver (Fig. 7A). However, unlike liver, metyrapone pretreatment greatly augmented IRS-1/PI3K association in skeletal muscle, to levels similar to the trauma only groups (Fig. 7B).
Figure 7.
Effects of metyrapone on the association between IRS-1 and PI3K in the liver and triceps. At the same time points and treatment regimens described in Fig. 5, the liver and triceps were removed and protein extracts were immunoprecipitated with specific total IRS-1 antibody and then subjected to Western blot analysis by specific total IRS-1 and anti-PI3K-p85 subunit antibodies. A and B, Representative blots are presented, respectively, from the liver and triceps (six rats each in the T0′, T60′, TH60′, and TH60′+veh groups, and 10 rats in the TH60′+Met group). IP, Immunoprecipitation; IB, immunoblot; Met, metyrapone; Veh, vehicle; Ins, insulin.
Thus, metyrapone inhibition of increased corticosterone levels, although ineffective in liver, greatly increased insulin-induced IRS-1/PI3K association and phosphorylation of Akt, IRS-1, and IR in skeletal muscle. This suggests that in muscle, glucocorticoids play an important role in the trauma and hemorrhage-induced insulin-resistant state.
Inhibition of corticosterone action prevented the development of insulin resistance in skeletal muscle, but not liver, after TH
RU486 induces or stabilizes an inactive glucocorticoid receptor conformation, and thereby acts as a glucocorticoid receptor antagonist (26,27). Therefore, RU486 was used to confirm that glucocorticoids are involved in the development of insulin resistance in skeletal muscle. Pretreatment with RU486 did not alter FFA levels or the increase in corticosterone levels after T or TH (Fig. 4, A and B). Levels of hepatic insulin-induced PS-Akt (Fig. 8, A and B) and PY-IRS-1 (Fig. 8, E and F), which were negligible after the combination of TH (TH60′), were unaltered by pretreatment with RU486. However, in triceps, insulin induction of PS-Akt (Fig. 8, C and D) and PY-IRS-1 (Fig. 8, G and H), which were negligible after the combination of trauma and hemorrhage, were mostly normalized by RU486. These results further demonstrate that the acute insulin resistance that develops after TH can be significantly improved by blocking glucocorticoid action in skeletal muscle, whereas there is little change in the development of insulin resistance in the liver.
Figure 8.
The inhibition of glucocorticoid receptor can increase Akt and IRS-1 phosphorylation in triceps but not the liver. RU486, a glucocorticoid receptor antagonist, was administered as described in Materials and Methods. Liver and triceps tissue lysates were subjected to Western blotting with specific anti-PS473-Akt and anti-PY612-IRS-1 antibodies. Representative blots are presented in A, C, E, and G. B, D, F, and H, Bars represent mean ± se from three to five rats in each group (five rats in TH60′+ins+RU486 group, four rats in TH60′-ins+RU486 group, three rats in other groups). Ins, Insulin; NS, not significant.
Discussion
Insulin resistance, in chronic conditions such as type 2 diabetes or obesity, has been extensively studied. However, almost nothing is known about the acute insulin-resistant state that occurs after major injury or infection. The cellular mechanisms have not been explored, and what insulin signaling pathway defects occur and in which tissues, are largely unknown. The two most extensively studied insulin signaling pathways are the IRS-1/PI3K/Akt and the Ras/MEK/ERK pathways (10). Our previous study demonstrated that insulin signaling through the Ras/MAPK kinase/ERK pathway remains relatively unchanged after trauma and hemorrhage in the liver (11). Therefore, the present study focused only on the acute impairment of the IRS-1/PI3K/Akt pathway. Although our previous work indicated that acute hepatic insulin resistance occurs in rats after TH (11,12,13), the present study examined much earlier time points and found that hepatic insulin resistance occurred much more rapidly than previously described or expected (11,12,13). Skeletal muscle is the major site of insulin-dependent glucose disposal, and changes in insulin signaling in skeletal muscle after hemorrhage have not been delineated. Thus, the development of skeletal muscle insulin resistance was investigated and was found to occur after hemorrhage but developed more slowly (at a later time) than that observed in liver.
Chronic insulin resistance is often associated with a marked increase in JNK activity in insulin-sensitive tissues (31,32,35). A novel finding of the present study is the tissue-specific difference in JNK activation, which was increased rapidly in liver after TH but not in skeletal muscle. Our findings (here and see Ref. 13) suggest a role for JNK in hepatic insulin resistance after hemorrhage but a lesser or no role in skeletal muscle. Activation of JNK is often coincident with increased IRS-1 PS312, a putative substrate of activated JNK (31,32). Although tyrosine phosphorylation of IRS-1 appears to be reduced in both liver and skeletal muscle after hemorrhage, IRS-1 PS312 is acutely increased in liver but difficult to measure in skeletal muscle, even when total cellular IRS-1 protein is easily measured. Therefore, the reduced tyrosine phosphorylation of IRS-1 in skeletal muscle is unlikely due to PS312, whereas in liver the induction of JNK activity and inhibition of IRS-1 function by serine phosphorylation are likely mechanisms contributing to the loss of insulin signaling.
The role of glucocorticoids in the development of insulin resistance has long been recognized (36,37). Usually it takes glucocorticoids several hours or days to induce insulin resistance in vivo and in vitro. However, other actions of glucocorticoids can occur more rapidly, sometimes within minutes (38,39,40). Glucocorticoids increase dramatically after trauma, hemorrhage, or infection (17). In the present work, the effects of glucocorticoid synthesis inhibition or glucocorticoid receptor antagonism on insulin signaling were used to elucidate the role of glucocorticoids in the development of acute insulin resistance after TH. Pretreatment with metyrapone or RU486 significantly rescued insulin-induced signaling via the IR/IRS-1/PI3K/Akt pathway in skeletal muscle but not liver. A surprising finding of the present study is that glucocorticoid-dependent insulin resistance in skeletal muscle occurs so rapidly, as early as 60 min after hemorrhage in skeletal muscle, much more rapidly than expected from previous studies (39,41,42). Our current data also suggests that systemic glucocorticoid concentrations increase before the development of skeletal muscle insulin resistance, which occurs between 30 and 60 min. In the liver, in which there is a more rapid development of insulin resistance, blockade of glucocorticoid action is not effective in blocking the acute development of insulin resistance.
The slower development of insulin resistance in skeletal muscle and the prevention of insulin resistance by inhibition of glucocorticoid synthesis or action in skeletal muscle, but not liver, in our animal model of acute injury and hemorrhage are reminiscent of the differences in glucocorticoid action previously published on the chronic actions of glucocorticoids. For instance, after 12 d of glucocorticoid treatment, insulin-induced phosphorylated Akt is greatly decreased in rat skeletal muscle, as is glucose uptake and disposal (22,39,41). However, insulin-stimulated Akt serine phosphorylation is not altered in liver of dexamethasone-treated rats. Within 5 d of glucocorticoid treatment, there is a reduction of insulin-inducible Akt activity in muscle but not liver, suggesting a muscle-specific role of glucocorticoids (43).
One possible explanation for the different roles of glucocorticoids between muscle and liver is the contributions of other factors that act differently in liver vs. muscle. For instance, increased glucocorticoids can stimulate lipolysis, which may increase FFA levels (44). Increased FFAs can stimulate the inhibitory serine phosphorylation of IRS-1 (45). However, this would not explain the role of glucocorticoids only in skeletal muscle after hemorrhage because there was no increase in PS-IRS-1 in skeletal muscle. In addition, FFAs were not increased by our TH procedure and were not altered by addition of either metyrapone or RU486. Thus, it is unlikely that FFAs play a role in the development of acute insulin resistance in skeletal muscle. Furthermore, it is unlikely that FFAs contribute to the development of insulin resistance in liver, even though there is an increase of inhibitory serine phosphorylation of IRS-1 in liver after hemorrhage.
Whereas elevated cortisol levels are essential for human survival in response to critical illness, increased glucocorticoids can also contribute to hyperglycemia and insulin resistance in critically ill patients. Several studies have demonstrated a positive correlation between increased serum cortisol levels, the severity of illness, and the risk of death (18,19,46). The present study clearly indicates that glucocorticoids are involved in the acute development of insulin resistance in skeletal muscle after hemorrhage. Thus, we suggest that increased glucocorticoids contribute to hyperglycemia and insulin resistance after injury but do so mainly by acting on skeletal muscle and inhibiting glucose disposal and not via altering hepatic gluconeogenesis. Whereas the liver becomes insulin resistant after hemorrhage, it is not via a glucocorticoid-dependent mechanism, and our recently published studies (13) indicate that TNFα plays a major role in the development of hepatic insulin resistance. If TNFα signaling is inhibited, there is a decrease in the hepatic levels of activated JNK and serine phosphorylation (inhibitory) of IRS-1.
In conclusion, we have characterized different tissue-specific mechanisms for the acute development of insulin resistance after injury in liver and skeletal muscle. Tissue-specific regulation of insulin signaling by glucocorticoids in skeletal muscle implies that inhibition of glucocorticoid action may offer a novel therapeutic strategy to reverse critical illness-induced skeletal muscle insulin resistance.
Acknowledgments
We thank the University of Alabama at Birmingham Clinical Nutrition Research Center and Dr. B. Gower for FFA and corticosterone measurements.
Footnotes
This work was supported by National Institutes of Health Grant DK 62071 and grants from the Veterans Administration Merit Review (to J.L.M.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 18, 2008
Abbreviations: FFA, Free fatty acid; IR, insulin receptor; IRS, insulin receptor substrate; JNK, c-Jun N-terminal kinase; PI3K, phosphatidylinositol 3-kinase; PS, phosphoserine; PY, phosphotyrosine; T, trauma only; TH, trauma and hemorrhage.
References
- Desouza C, Gilling L, Fonseca V 2001 Management of the insulin resistance syndrome. Curr Diab Rep 1:140–147 [DOI] [PubMed] [Google Scholar]
- Kendall DM, Harmel AP 2002 The metabolic syndrome, type 2 diabetes, and cardiovascular disease: understanding the role of insulin resistance. Am J Manag Care 8:S635–S653 [PubMed] [Google Scholar]
- Ikezu T, Okamoto T, Yonezawa K, Tompkins RG, Martyn JA 1997 Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J Biol Chem 272:25289–25295 [DOI] [PubMed] [Google Scholar]
- Carter EA 1998 Insulin resistance in burns and trauma. Nutr Rev 56:S170–S176 [DOI] [PubMed] [Google Scholar]
- Lange MP, Dahn MS, Jacobs LA 1985 The significance of hyperglycemia after injury. Heart Lung 14:470–472 [PubMed] [Google Scholar]
- Nelson KM, Filkins JP 1979 Effect of traumatic injury on sensitivity to insulin. Circ Shock 6:285–295 [PubMed] [Google Scholar]
- Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R 2001 Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367 [DOI] [PubMed] [Google Scholar]
- van den BG, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, Bouillon R, Schetz M 2006 Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes 55:3151–3159 [DOI] [PubMed] [Google Scholar]
- White MF1997 The insulin signalling system and the IRS proteins. Diabetologia 40(Suppl):S2–S17 [DOI] [PubMed] [Google Scholar]
- Nakae J, Accili D 1999 The mechanism of insulin action. J Pediatr Endocrinol Metab 12(Suppl 3):721–731 [PubMed] [Google Scholar]
- Ma Y, Wang P, Kuebler JF, Chaudry IH, Messina JL 2003 Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol 284:G107–G115 [DOI] [PubMed] [Google Scholar]
- Ma Y, Toth B, Keeton AB, Holland LT, Chaudry IH, Messina JL 2004 Mechanisms of hemorrhage-induced hepatic insulin resistance: role of tumor necrosis factor-α. Endocrinology 145:5168–5176 [DOI] [PubMed] [Google Scholar]
- Xu J, Kim HT, Ma Y, Zhao L, Zhai L, Kokorina N, Wang P, Messina JL 2008 Trauma and hemorrhage-induced acute hepatic insulin resistance: dominant role of tumor necrosis factor (TNF)-α. Endocrinology 149:2369–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR 2006 From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 68:123–158 [DOI] [PubMed] [Google Scholar]
- Qi D, Rodrigues B 2007 Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab 292:E654–E667 [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Herzig S 2007 Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol 275:43–61 [DOI] [PubMed] [Google Scholar]
- Vaughan GM, Becker RA, Allen JP, Goodwin Jr CW, Pruitt Jr BA, Mason Jr AD1982 Cortisol and corticotrophin in burned patients. J Trauma 22:263–273 [DOI] [PubMed] [Google Scholar]
- Jurney TH, Cockrell Jr JL, Lindberg JS, Lamiell JM, Wade CE 1987 Spectrum of serum cortisol response to ACTH in ICU patients. Correlation with degree of illness and mortality. Chest 92:292–295 [DOI] [PubMed] [Google Scholar]
- Span LF, Hermus AR, Bartelink AK, Hoitsma AJ, Gimbrere JS, Smals AG, Kloppenborg PW 1992 Adrenocortical function: an indicator of severity of disease and survival in chronic critically ill patients. Intensive Care Med 18:93–96 [DOI] [PubMed] [Google Scholar]
- Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR 1998 Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab 83:757–760 [DOI] [PubMed] [Google Scholar]
- Vanhorebeek I, Peeters RP, Vander PS, Jans I, Wouters PJ, Skogstrand K, Hansen TK, Bouillon R, van den BG 2006 Cortisol response to critical illness: effect of intensive insulin therapy. J Clin Endocrinol Metab 91:3803–3813 [DOI] [PubMed] [Google Scholar]
- Giorgino F, Almahfouz A, Goodyear LJ, Smith RJ 1993 Glucocorticoid regulation of insulin receptor and substrate IRS-1 tyrosine phosphorylation in rat skeletal muscle in vivo. J Clin Invest 91:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad MJ, Folli F, Kahn JA, Kahn CR 1993 Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest 92:2065–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Le MM, Piazza PV 1996 Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res 724:251–255 [DOI] [PubMed] [Google Scholar]
- Sillence MN, Rodway RG 1987 Effects of metyrapone and etomidate on adrenal function and growth rate in female rats. J Endocrinol 113:473–478 [DOI] [PubMed] [Google Scholar]
- Donley MP, Schulkin J, Rosen JB 2005 Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res 164:197–205 [DOI] [PubMed] [Google Scholar]
- Blais V, Zhang J, Rivest S 2002 In altering the release of glucocorticoids, ketorolac exacerbates the effects of systemic immune stimuli on expression of proinflammatory genes in the brain. Endocrinology 143:4820–4827 [DOI] [PubMed] [Google Scholar]
- Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM 2004 Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol 97:1329–1337 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP 1981 The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS 1999 Mechanisms of TNF-α-induced insulin resistance. Exp Clin Endocrinol Diabetes 107:119–125 [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB 2006 Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF 2002 Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277:1531–1537 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM 1996 IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in tumor necrosis factor-α- and obesity-induced insulin resistance. Science 271:665–668 [DOI] [PubMed] [Google Scholar]
- Boden G 1997 Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46:3–10 [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS 2002 A central role for JNK in obesity and insulin resistance. Nature 420:333–336 [DOI] [PubMed] [Google Scholar]
- Nosadini R, Del PS, Tiengo A, Valerio A, Muggeo M, Opocher G, Mantero F, Duner E, Marescotti C, Mollo F, Belloni F 1983 Insulin resistance in Cushing’s syndrome. J Clin Endocrinol Metab 57:529–536 [DOI] [PubMed] [Google Scholar]
- Pagano G, Cavallo-Perin P, Cassader M, Bruno A, Ozzello A, Masciola P, Dall'omo AM, Imbimbo B 1983 An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest 72:1814–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnert C, Ruchat S, Nicod N, Tappy L 2004 Dexamethasone-induced insulin resistance shows no gender difference in healthy humans. Diabetes Metab 30:321–326 [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Wagman AS, Jensen J 2005 Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia 48:2119–2130 [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M 2000 Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol Rev 52:513–556 [PubMed] [Google Scholar]
- Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, Krause U, Bevan S, Piva T, Wegener G, Newsholme EA 1997 Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J 321(Pt 3):707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corporeau C, Foll CL, Taouis M, Gouygou JP, Berge JP, Delarue J 2006 Adipose tissue compensates for defect of phosphatidylinositol 3′-kinase induced in liver and muscle by dietary fish oil in fed rats. Am J Physiol Endocrinol Metab 290:E78–E86 [DOI] [PubMed] [Google Scholar]
- Le FC, Corporeau C, Le Guen V, Gouygou JP, Berge JP, Delarue J 2007 Long-chain n-3 polyunsaturated fatty acids dissociate phosphorylation of Akt from phosphatidylinositol 3′-kinase activity in rats. Am J Physiol Endocrinol Metab 292:E1223–E1230 [DOI] [PubMed] [Google Scholar]
- Slavin BG, Ong JM, Kern PA 1994 Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res 35:1535–1541 [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI 2002 Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E 2000 A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 283:1038–1045 [DOI] [PubMed] [Google Scholar]