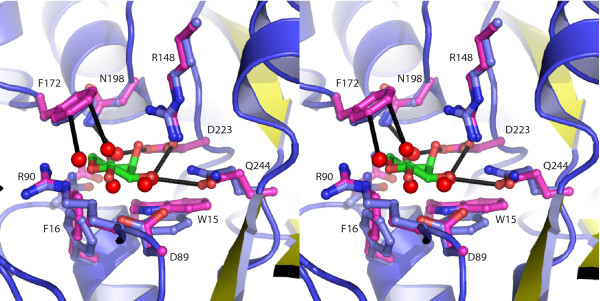
Figure 7.
Binding pocket organization of the apo and ribose-bound tmRBP. Stereo-view of the ribose-bound tmRBP (blue) binding pocket superimposed with the binding pocket amino acids of apo tmRBP (magenta). The C-terminal residues of the apoprotein have similar rotamers as the ribose-bound form while the rotamers of the N-terminal domain apoprotein and ribose-bound forms are in different states. The C-terminal binding pocket residues of the apoprotein interact (black lines) with bulk solvent (red spheres) in a similar manner as the ligand-bound form does with the ribose ligand, pre-organizing the apo form.