Abstract
The localization of proteins by immunostaining is a powerful method to investigate otologic disorders. However, the use of fixatives and embedding media (necessary for preservation of morphology) can obscure antigens, making it difficult to perform immuno assays. We performed a systematic investigation of effects of the fixative and embedding medium on morphology and immunostaining of the mouse cochlea. Three different fixative solutions- 4% formaldehyde (F), 4% formaldehyde + 1% acetic acid (FA), and 4% formaldehyde + 1% acetic acid + 0.1% glutaraldehyde (FGA); and three different embedding media- paraffin, polyester wax, and celloidin were used. Morphology was assessed using light microscopy. Immunostaining was studied using a panel of six antibodies (to prostaglandin D synthase, aquaporin 1, connective tissue growth factor, 200 kD neurofilament, tubulin and Na+,K+- ATPase).
Preservation of morphology was suboptimal with paraffin, adequate with polyester wax and superb with celloidin. Immunostaining was successful using all six antibodies in all three fixatives and all three embedding media. While there were differences in strength of signal and localization of antigen between the three fixatives, overall, FA and FGA gave the most uniform results. For a given fixative and antibody, there was surprisingly little difference in the quality of immunostaining between celloidin and paraffin, while results in polyester wax were not as good in some cases. These results suggest that celloidin may be the embedding medium of choice for both morphological and pathological studies, including immunostaining when morphology must be optimized.
Keywords: cochlea, morphology, immunostaining, celloidin, temporal bone
Introduction
Knowledge of the pathologic basis of disease is central to the study of medicine including disorders affecting the auditory and vestibular systems. Otology is unique in that the inner ear is inaccessible during life, so that conventional techniques of pathologic studies such as biopsy and surgical excision are not feasible. Hence, insight into the pathologic basis of ear disease can be obtained only by postmortem study of human temporal bones and by developing credible animal models. The human temporal bone poses unique challenges for microscopic study, by virtue of containing the delicate membranous labyrinth suspended in fluid but encased in an extremely dense shell of otic capsule bone.
A mainstay of otopathologic study has been examination of morphology using light microscopy after fixation, decalcification, embedding and serial sectioning [Schuknecht, 1993]. A variety of fixatives and embedding media have been described and used by investigators over the past 100 years. The goal of tissue fixation is to stop autolysis and preserve cellular architecture. Fixation also protects cells against the harsh chemicals necessary for processing and embedding [Skepper, 2000]. Over the past few decades, immunohistochemical staining has proven to be a valuable tool for the otologic researcher. The goal of immunostaining is to enable specific recognition of an antigen and binding of an antibody to that antigen. The goals of tissue fixation and immunostaining are at odds because crosslinking of proteins by fixatives can inhibit antigen-antibody recognition. In addition, the embedding medium can result in background staining or further mask an antigen, producing a weak signal. Although immunostaining can be instructive under normal and pathologic conditions [e.g., Keithley et al. 1995; Ganbo et al. 1997; Adams 2002; Zehnder et al. 2005; Lopez et al. 2005], getting to this point is challenging in temporal bone tissue.
Many different factors can affect the outcome of morphologic study and the ability to immunostain temporal bone sections, including the fixative, decalcifying agent, embedding medium, duration of postmortem time, properties specific to the particular antibody under investigation, and effects of pathology or disease on the tissue being studied. Many of these variables interact with one another to affect the ultimate outcome. There are no published reports describing systematic investigations of how these variables might affect the outcome of temporal bone research in terms of morphology and immunostaining.
Our ultimate goal is to optimize techniques of study that would permit study of the human temporal bone while preserving morphologic detail and ease of immunostaining. We wish to develop a resource of protocols by systematically studying how the variables described above affect morphology and immunostaining ability. Such knowledge would permit us to glean the most information from every specimen. However, it is difficult to conduct such a systematic study using human specimens. One problem is the difficulty in controlling some of the relevant variables in human tissue (e.g. postmortem time or effect of disease). Another problem is the general paucity of human specimens, both normal and pathologic. Therefore, we chose to systematically explore these questions in mouse tissue where pertinent variables can be controlled. In the present study, we systematically studied the effects of fixative and embedding medium on morphology and the ability to immunostain using a panel of six different antibodies. We chose 3 commonly used fixatives (formaldehyde [F], formaldehyde + acetic acid [FA], and formaldehyde + acetic acid + glutaraldehyde [FGA]) and 3 commonly used embedding media (paraffin, polyester wax and celloidin).
Material and Methods
This study was approved by our Institutional Animal Care Committee. Fifteen CBA/CaJ mice ranging in age from 4 weeks to 12 weeks were deeply anesthetized and intracardially perfused with one of 3 fixatives, 4% formaldehyde (F), 4% formaldehyde + 1% acetic acid (FA), and 4% formaldehyde + 1% acetic acid + 0.1% glutaraldehyde (FGA). The temporal bones were removed and fixed for an additional 25 hours at room temperature. Decalcification was accomplished using 120 mM ethylenediaminetetraacetic acid at a pH of 7 for seven days. The temporal bones were rinsed in distilled water and dehydrated in ethanols. In nine of the fifteen mice, the left ears were embedded in polyester wax (Electron Microscopy Sciences, Fort Washington, PA) and the right ears were embedded in celloidin (parlodion strips) (Mallinckrodt Chemicals, Phillipsburg, NJ). Both ears of the remaining six mice were embedded in paraffin (Paraplast X-TRA) (McCormick Scientific, St. Louis, MO). Temporal bones were sectioned at a thickness of 8-20 μm depending on the embedding medium. Paraffin sections were sectioned at 8 μm thickness and mounted on superfrost plus slides. Polyester wax sections were sectioned at 10 μm and mounted on superfrost slides coated with 0.5% bovine albumin and 0.5% fish gelatin [Merchant et al., 2006]. Celloidin sections were sectioned at 20 μm and those being immunostained were mounted on subbed glass slides smeared with albumin and fixed in place with formalin-soaked bibulous paper. A wooden block was placed on top of the bibulous paper and a 500g weight was placed on the block. The sections were dried for 1 hour under the weight.
Every tenth section was stained with hematoxylin and eosin and examined by light microscopy. Preservation of morphology within the scala media of the cochlea was assessed for each of the three fixatives and each of the three embedding media.
Prior to immunostaining, selected paraffin sections were dewaxed using xylenes, polyester wax was removed using ethyl alcohols [Merchant et al., 2006], and celloidin was removed using a solution of sodium hydroxide mixed in methanol [Miguel-Hidalgo and Rajkowska, 1999]. The sodium hydroxide methanol (NaOH-methanol) solution was made in the same manner as that described by Miguel-Hidalgo and Rajkowska; however the subsequent steps were modified. Once mixed, the solution was diluted 1:2 with methanol instead of 1:3 and used immediately. The amount of time the sections were exposed to the NaOH-Methanol solution was reduced from 30 minutes to 15 minutes (3 × 5 minutes) with 100% methanol rinses between each 5 minute change. The 3% H2O2step described by Miguel-Hidalgo and Rajkowska was omitted. The sections were hydrated from 100% methanol (10 minutes), through 70% methanol (10 minutes), and into distilled water. Following hydration, the sections were rinsed in 0.01 M phosphate buffered saline (PBS, pH 7.3), incubated in 5% normal horse serum for one hour, then incubated in primary antibodies overnight at room temperature in a humid chamber.
Immunostaining was accomplished using antibodies to prostaglandin d synthase (PGDS, Caymen) at a dilution of 1:5000, aquaporin 1 (Aqp1, Chemicon) at a dilution of 1:1000, connective tissue growth factor (CTGF, Cell Sciences) at a dilution of 1:10,000, 200 kD neurofilament (NF, Boehringer Mannheim) at a dilution of 1:2000, tubulin (Sigma) at a dilution of 1:15,000, and Na+,K+-ATPase (Siegel) at a dilution of 1:10,000. Following 14 hour primary antibody incubations, sections were rinsed in three washes of PBS. Secondary antibodies appropriate for the host species of primaries, at a dilution of 1:200, were applied and incubated for 1 hour. Following another three rinses in PBS, avidin-biotin-horseradish peroxidase (Standard ABC Kit, Vector Labs, Burlingame, CA) was applied to the sections and left to incubate for 1 hour, followed again by three washes with PBS. Finally, the sections were colorized using 0.01% diaminobenzidine and 0.01% H2O2 for between 5 and 10 minutes, rinsed, dehydrated, and cover slips were applied. Immunostaining was repeated at least once with each antibody, and in most cases, multiple times. The immunostained sections were analyzed by light microscopy and given a rating of no staining (−), adequate staining (+), good staining (+ +), or very good staining (+ + +).
Results
Preservation of Morphology
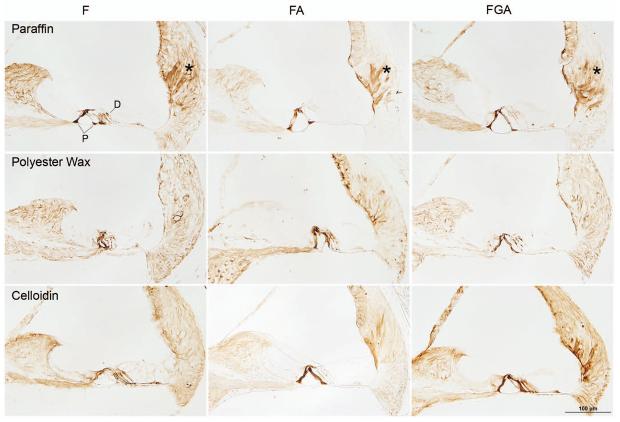
Fig. 1 demonstrates representative images of the scala media using each of the three fixatives and the three embedding media.
Fig. 1. Preservation of morphology: showing effect of fixative verses embedding medium (hematoxylin and eosin stain).
Paraffin (top panel): Morphology is less than optimal with paraffin, regardless of fixative used. Morphology of cells in the inner sulcus, outer sulcus (asterisk) and of supporting cells is poor with formalin alone. The morphology is somewhat improved with FA and FGA, but it is not as good as the other embedding media. Paraffin embedding also results in multiple breaks and folding of membranes such as Reissner's membrane (filled arrow) and the basilar membrane (arrowhead), as well as distortion of the interscalae septa (not shown).
Polyester wax (middle panel): Although subtle, morphologic preservation of the organ of Corti is improved in polyester wax compared to paraffin. There is progressive improvement in morphology from F to FA to FGA, this improvement is evident in the outer sulcus (asterisk), for example. There is a background eosinophilic staining (arrow) in the polyester wax sections which is caused by the coating of gelatin and albumin on the slide. There is also a tendency for membrane breaks to occur, although less so than in paraffin.
Celloidin (bottom panel): Celloidin embedding shows the best morphology, which is revealed with superb preservation of all cells including the inner and outer sulcus cells as well as the organ of Corti. Membrane breaks are rare with celloidin embedding. Note the tendency in improved morphology as one goes from F, to FA, to FGA (for example, the inner hair cell area). F=formalin, FA=formalin + acetic acid, FGA = formalin + glutaraldehyde + acetic acid.
Paraffin embedding gave less than optimal morphology, regardless of the fixative used. As can be seen in the top panel of Fig. 1, preservation of the cells within the inner and outer sulci was poor with F. Although morphology improved with FA and FGA, it remained sub-optimal. Artifactual breaks and folding of edges occurred frequently within the membranous labyrinth (e.g., Reissner's membrane, basilar membrane, interscalar septum, etc).
Polyester wax gave somewhat better preservation than paraffin. Morphology was better with FA or FGA compared to F alone. This was particularly evident when examining cells of the outer sulcus as shown in the middle panel of Fig. 1. There was a tendency for membrane breaks to occur, especially in Reissner's membrane, although less so than with paraffin. The polyester wax protocol caused a background eosinophilic staining as a result of the gelatin-albumin coating on the slide.
Celloidin embedding gave the best morphology of all three embedding media. Membrane breaks were rare with celloidin. In general, FA and FGA showed the best resolution of anatomical detail.
Immunostaining
Table 1 demonstrates the results for each antibody, comparing fixative to embedding medium. Immunostaining was successfully achieved using all 6 antibodies in all 3 fixatives and all 3 embedding media (Figures 2-5). As described before, immunostaining was repeated at least twice with each antibody; results were consistent and similar with repeat staining for all antibodies. However, degree of success varied, depending upon the antibody, fixative and embedding medium.
Table 1.
Scoring for type of fixative versus embedding media for panel of six antibodies based on light microscopic examination: - no staining, +adequate staining, + + good staining, + + + very good staining
| PGDS | Formalin | FA | FGA |
|---|---|---|---|
| Paraffin | + + | + + + | + + + |
| Polyester wax | + | + + | + + + |
| Celloidin | + | + + + | + + + |
| Aqp1 | |||
| Paraffin | + + + | + + + | + + + |
| Polyester wax | + | + + | + + + |
| Celloidin | + | + + + | + + + |
| CTGF | |||
| Paraffin | + + | + + | + + |
| Polyester wax | + + | + | + + |
| Celloidin | + + | + + | + + + |
| NF | |||
| Paraffin | + + | + + + | + + + |
| Polyester wax | + + | + + + | + + + |
| Celloidin | + + + | + + + | + + |
| Tubulin | |||
| Paraffin | + + + | + + + | + + + |
| Polyester wax | + | + + | + |
| Celloidin | + | + + | + + + |
| Na+,K+-ATPase | |||
| Paraffin | + + + | + + | + + + |
| Polyester wax | + | + + | + |
| Celloidin | + | + + | + + + |
Fig. 2. Immunostaining for PGDS.
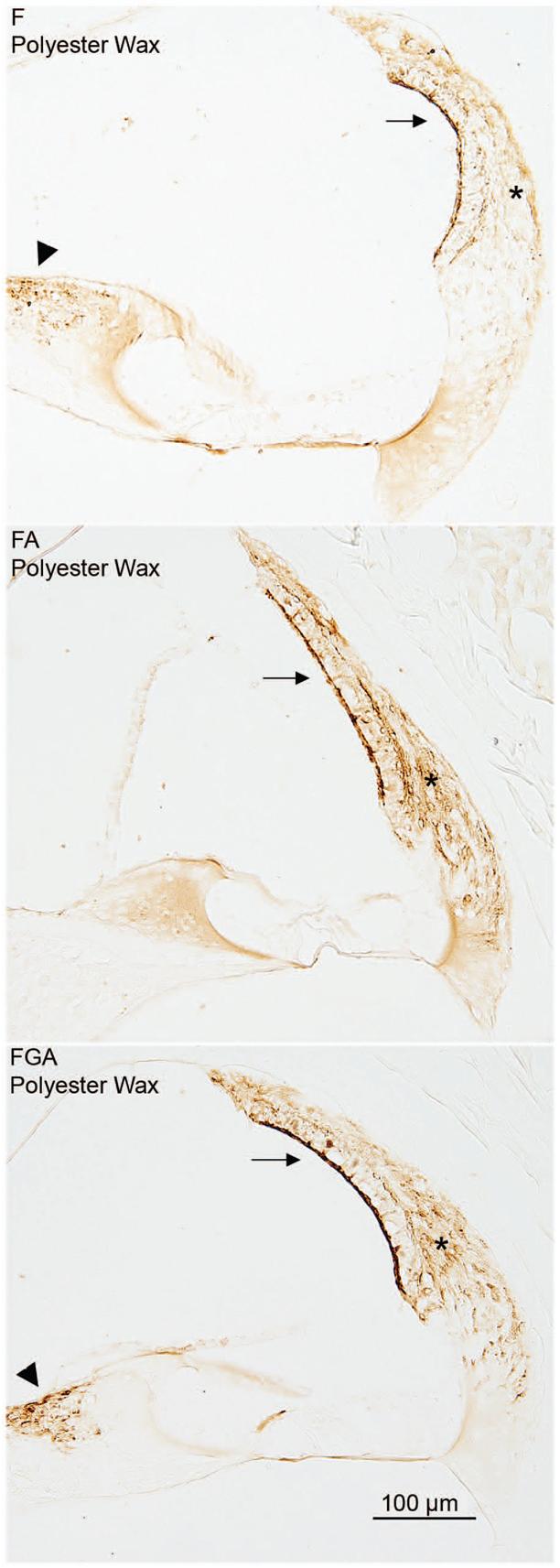
The effect of fixative is demonstrated in this panel. The PGDS antibody stains the fibrocytes of the spiral limbus (arrowhead), the type I fibrocytes (asterisk) of the spiral ligament and the marginal cells of the stria vascularis (arrow). In all three images shown in the panel, the embedding medium was polyester wax. The immunostaining improves as one progresses from the F fixed tissue to the FA tissue to the FGA tissue.
Fig. 5. Immunostaining for Na±, K±-ATPase, CTGF, and Aqp1.
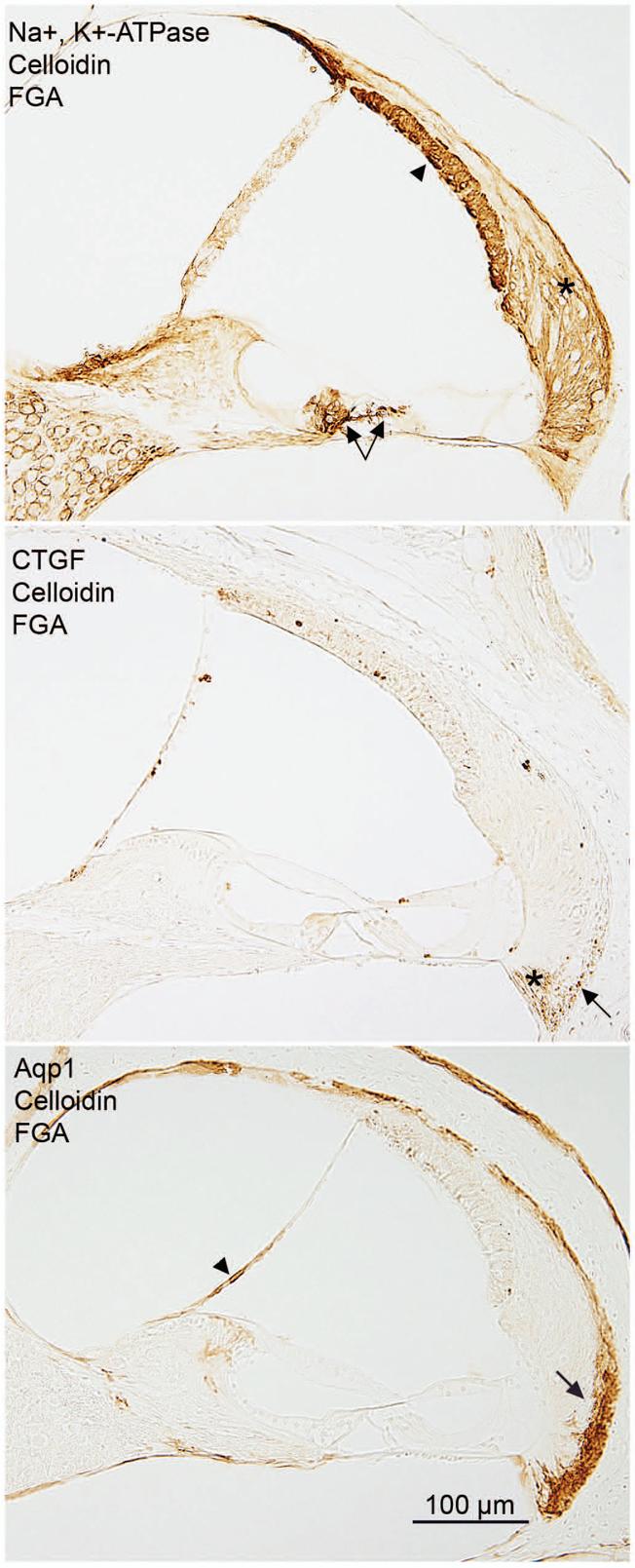
The three panels in this figure show representative staining patterns for Na+,K+-ATPase, CTGF, and Aqp1 antibodies when the tissue is fixed with FGA and embedded in celloidin. The Na+, K+-ATPase antibody stains the stria (arrowhead), the type II fibrocytes of the spiral ligament (asterisk), and nerve fibers below the inner and outer hair cells (arrows). The CTGF antibody stains the type IV fibrocytes of the spiral ligament (asterisk) and some type III fibrocytes of the spiral ligament (arrow). The bottom photo demonstrates a typical staining pattern for the Aqp1 antibody. The type III fibrocytes of the spiral ligament are stained (arrow) as well as the medial portion of Reissner's membrane (arrowhead).
The PGDS antibody stained the strial marginal cells, the type I fibrocytes of the spiral ligament, and some fibrocytes of the spiral limbus. For a given fixative, immunostaining was about the same regardless of embedding medium. On the other hand, for a given embedding medium, FA and FGA gave superior immunostaining compared to F alone. Figure 2 demonstrates the progressive improvement in immunostaining with the PGDS antibody in polyester wax when comparing F to FA to FGA. Formalin fixation also produced inferior results with this antibody in paraffin and celloidin sections (Table 1).
The Aqp1 antibody stained the type III fibrocytes of the spiral ligament and the medial portion of Reissner's membrane (Fig. 5). The FGA fixative was the best overall, and was rated very good in all three embedding media. The F-fixed tissue rated adequate when embedded in polyester wax and celloidin, and very good in paraffin. The FA-fixed tissue rated good or very good.
The CTGF antibody stained the type IV fibrocytes of the spiral ligament (Fig. 5), some type III fibrocytes of the spiral ligament (Fig. 5), Deiter's cells in the apical turn of the cochlea, and sometimes the root cells. The best staining for CTGF was achieved in the FGA fixed tissue embedded in celloidin. Good staining was possible with F, FA, and FGA in paraffin as well as F and FGA fixed and polyester wax embedded. Good staining for CTGF was also achieved in F and FA fixed tissue embedded in celloidin. Adequate staining was possible in FA fixed tissue embedded in polyester wax.
The NF antibody stained nerve fibers beneath the inner hair cells, tunnel crossing fibers, and nerve fibers below the outer hair cells (Figure 3). The FA fixative produced very good staining in all three embedding media. Very good staining was also achieved with formalin in celloidin, and FGA in polyester wax and paraffin. Good staining was possible with F in paraffin and polyester wax, as well as FGA fixed in celloidin. Figure 3 demonstrates that with the NF antibody, good immunostaining was achieved regardless of embedding medium or fixative.
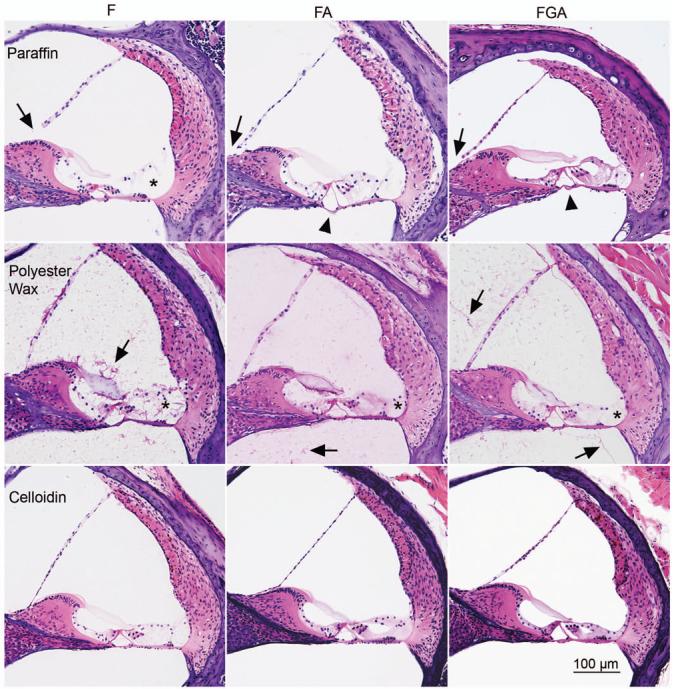
Fig. 3. Immunostaining for NF.
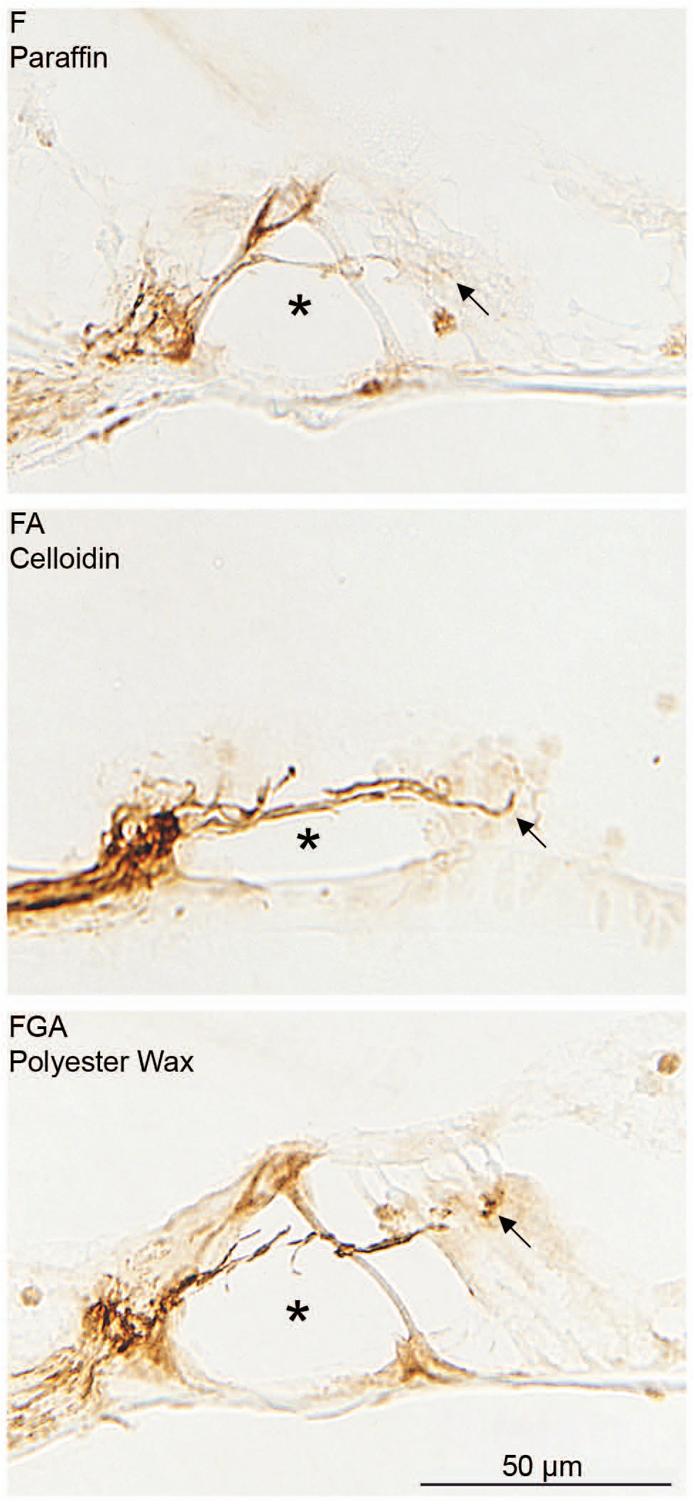
Antibodies against NF appear to stain just as well regardless of the fixative or the embedding media. The antibody stains nerve fibers underneath the hair cells (arrow) and those crossing the tunnel of Corti (asterisk). Good immunostaining is seen regardless of fixative or embedding medium. The section in the middle appears to be staining more completely or darker than the other two. However, it is embedded in celloidin and is thicker (20 microns). It also has better preservation. These factors may account for the apparent darker staining.
The tubulin antibody stained the pillar cells, Deiter's cells, root cells and fibrocytes of the spiral ligament (Fig. 4). Paraffin sections demonstrated the best staining for tubulin overall, being very good regardless of the fixative. Polyester wax sections showed adequate or good staining, but in no case were the root cells stained well. The effect of fixative was most conspicuous in the celloidin sections with progressive improvement in staining from F to FA to FGA.
Fig. 4. Immunostaining for tubulin.
These panels demonstrate the effect of fixative as well as embedding medium. The paraffin sections demonstrate the best overall staining for tubulin. The antibody to tubulin stains the pillar cells (P), Deiter's cells (D) and root cells (asterisk). Staining of the root cells is not evident in polyester wax sections. In the case of celloidin, there is an effect of fixation with progressive improvement in staining of root cells from F to FA to FGA.
The Na+,K+-ATPase antibody stained the type II fibrocytes of the spiral ligament, the marginal cells of the stria vascularis and nerve fibers below the inner and outer hair cells (Fig.5). The FA fixative rated good in all three embedding media. The F and FGA fixed tissue scored very good when embedded in paraffin, as did the FGA fixed tissue when embedded in celloidin.
Discussion
Preservation of morphology was influenced by both the fixative and the embedding medium. While formalin gave adequate fixation, morphology was improved with addition of acetic acid or glutaraldehyde plus acetic acid. Regardless of the fixative used, paraffin gave suboptimal preservation of anatomical detail. Preservation of morphology was adequate in polyester wax. The most superior preservation was observed with celloidin embedding.
The morphological differences between celloidin, polyester wax, and paraffin are more obvious in human specimens (versus mouse). Figure 6 shows typical examples of human bones in our collection processed in these 3 embedding media. Celloidin clearly gives the best preservation while paraffin is the worst. The differences are most evident in the organ of Corti, tectorial membrane, basilar membrane, and Reissner's membrane. The greater contrast in results in humans may be attributed to the fact that tissue preservation in humans is additionally degraded by factors such as postmortem autolysis.
Fig. 6. Human tissue embedded in celloidin, polyester wax and paraffin.
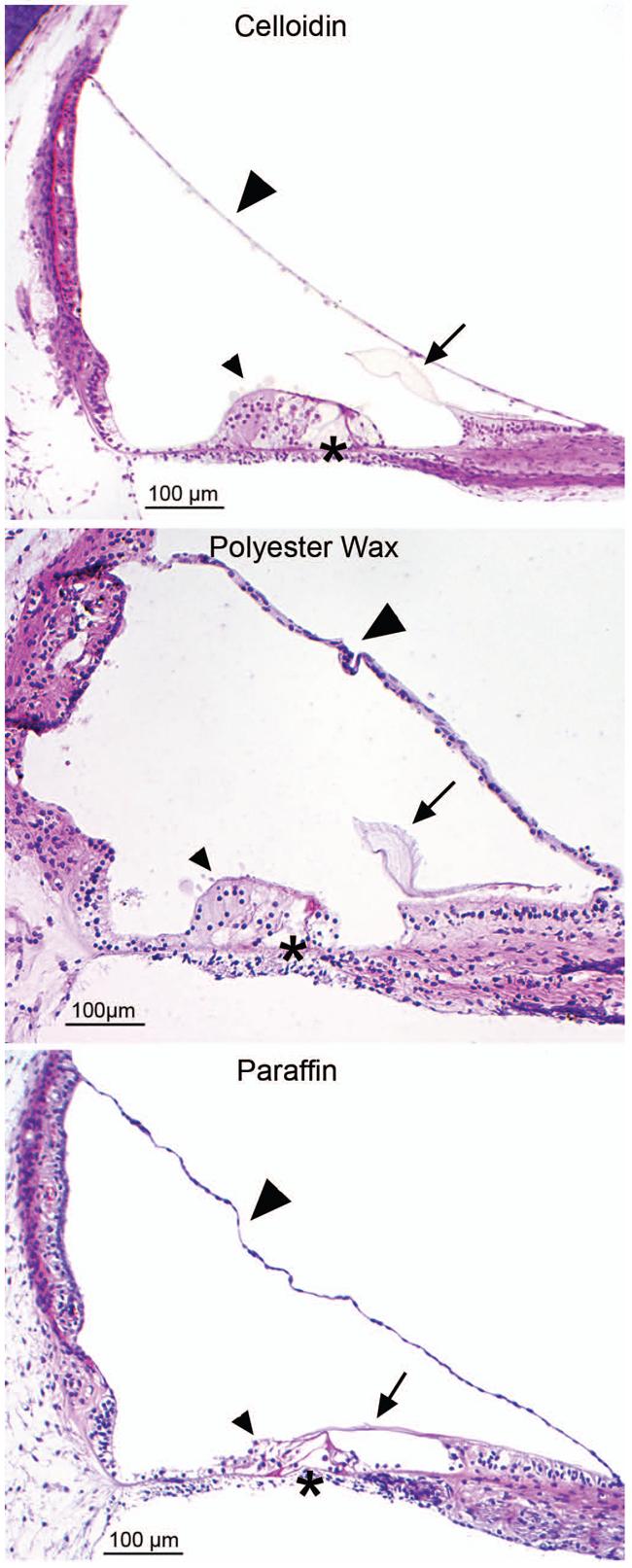
Differences in morphological preservation can be expected in human tissue processed in celloidin, polyester wax, and paraffin. The celloidin section, on the top, is from a 63 year old female, postmortem time was 7.5 hours. The polyester wax section in the middle is from a 71 year old female, postmortem time was 14 hours. The paraffin section, on the bottom, is from a 3 month old, postmortem time was 1.5 hours. The tectorial membrane (arrows) is shrunken in the paraffin compared to the celloidin. The integrity of the organ of Corti (small arrowheads) is much better in the celloidin section, fairly good in the polyester wax section, and shrunken and broken in the paraffin tissue. Also note the waviness of the basilar membrane (asterisks) in the paraffin section versus the polyester wax and celloidin sections. Reissner's membrane (large arrowheads) is also wavy in both the polyester wax section and the paraffin section, but not in the celloidin section.
Immunostaining of the mouse cochlea was successful with all six antibodies using all three fixatives and all three embedding media. The antibodies were chosen to stain a wide variety of cell types. The findings were in keeping with previous reports in applicable cases [Hafidi et al., 1990, Schulte and Adams, 1989, Slepecky and Ulfendahl, 1992, Stankovic et al., 1995]. Although one fixative and embedding medium will never be ideal for all potential antibodies, overall, we found that FA and FGA gave consistent staining in all three media using these six antibodies. However, there were differences in the quality of immunostaining between the three fixatives and three media.
The FA fixed tissue often showed more selective staining than formaldehyde alone. Adding acetic acid, a known denaturing agent, may alter protein shape or tertiary structure, thereby potentially unmasking the binding sites of some antigens. However, if the epitope being stained requires spatial organization for proper recognition, then denaturing the protein could prevent appropriate binding. Therefore, it is important to establish the optimal fixative for a particular antibody. Traditionally, acetic acid was added to fixatives such as Carnoy's and Clarke's; these latter fixatives contain alcohol which tends to shrink cells. It is believed that the acetic acid causes cells to swell, thus counteracting the effects of the alcohol [Luna, 1992]. There was no ethanol or methanol in the fixatives used in the present study, but the acetic acid appeared to improve both morphology and immunostaining. Acetic acid is also believed to penetrate cells rapidly [Luna, 1992], so perhaps, the acid in FA was facilitating faster fixation or more complete fixation.
The addition of glutaraldehyde to a fixative must be thought about in the opposite manner to addition of acetic acid. Glutaraldehyde is a stronger fixative, causing robust inter- and intra-molecular crosslinking of proteins [Hassell and Hand, 1974]. It is the fixative of choice for electron microscopy because it preserves fine ultrastructural detail. Some antigens, especially those in high quantity and those in secretory vesicles, withstand glutaraldehyde fixation very well and still maintain their ability to specifically bind their corresponding antibodies. For other proteins, even short exposure to a fixative as strong as glutaraldehyde can inhibit binding to their respective antibodies [Hassell and Hand, 1974].
In addition to fixatives, embedding media can also influence antibody penetration, binding and interpretation of results. Of the three embedding media used in this study, paraffin tissue scored highest overall for immunostaining. The heat involved in paraffin processing may be denaturing proteins and allowing better antigen-antibody recognition. High temperature is believed to cleave the cross-links formed by formaldehyde fixation [Yamashita and Okada, 2005]. Shi suggested that heat can induce “a re-naturation” of proteins [Shi et al., 2001], although it is debatable whether proteins can resume their tertiary structure following treatment with heat. In any case, heat is probably responsible for the inferior tissue preservation seen in paraffin embedded sections. Thus, the enhanced antibody penetration in paraffin embedded tissue comes at the cost of preservation of morphology. If superior morphology is necessary, celloidin is a better choice as an embedding medium.
The morphology of the polyester wax embedded tissue was somewhat superior to paraffin embedding, but immunostaining with polyester wax was suboptimal compared to both paraffin and celloidin. There maybe several reasons for these mixed results with polyester wax. We employed a mixture of bovine albumin and fish gelatin to adhere the polyester wax sections to the slides. The additional protein in the solution may have caused interference with antigen-antibody recognition. Incomplete removal of the polyester wax may also have contributed to decreased immunostaining. Another factor may be that polyester wax sections did not go through treatment with heat (as in paraffin) or with sodium hydroxide-methanol (as with celloidin). Heat and pH are factors known to be important for successful immunostaining in formalin fixed and embedded material. Heat and extremes of pH (either high or low) are believed to promote cleavage of cross links of methylene bridges formed by formaldehyde [Yamashita and Okada, 2005].
The celloidin sections stained well with all six antibodies. These results were somewhat surprising in light of our previous experience and that of others [e.g., Tian et al., 1999] that immunostaining human temporal bone sections embedded in celloidin is fraught with difficulty and inconsistency of results. We believe that our success may have been critically dependent on the sodium hydroxide-methanol method that we used for removing celloidin. This technique appears to achieve complete removal of celloidin, which may have made the antigens more accessible to the antibodies. Other techniques of celloidin removal that have been described include the use of clove oil [Portmann et al., 1990], acetone [Tian et al., 1999] and ether alcohol [Miguel-Hidalgo and Rajkowska, 1999]. We intend to investigate these other techniques in a future study. Nevertheless, our results indicate that there is no reason to alter the medium that is generally recognized as providing the best preservation of temporal bones in order to immunostain them.
As already noted, a factor that affects the quality of immunostaining is the time between death and fixation of the tissue (the postmortem time). The present study utilized tissue that underwent vascular perfusion to minimize the amount of diffusion time necessary for the fixatives to reach the cells. In other words, our tissue was fixed optimally. We plan on carrying out experiments in mouse ears using varying postmortem times to better approximate what occurs in human temporal bone specimens. Immunostaining mouse celloidin sections reliably and repeatedly is necessary for future work in human specimens. Determining the parameters that affect immunostaining in perfused tissue is the first step in beginning to sort out what can be expected in human material. Only under the most favorable conditions, can we begin to build a profile of what are reliable staining patterns for each antibody used. The utility of the work presented here is perhaps the opportunity to efficiently immunostain some of the thousands of human celloidin sections stored in 80% ethanol in various laboratories. Armed with the knowledge of the results presented here, combined with future work in mice that are not perfused, but subject to postmortem times, we are hopeful that conditions affecting each antibody tested will begin to become apparent and be instructive about what staining patterns can be expected in human tissue.
In addition, we point out that success with immunostaining depends not only on the many variables described above, but also on the idiosyncrasies of antigens and antibodies. Prior to immunostaining human temporal bone sections, it is advisable to optimize the staining protocol in animal tissue using conditions and parameters that approximate the constraints imposed by the particular human tissue at hand. In this manner, a researcher can maximize the information that can be extracted from scarce and valuable human specimens.
Conclusions
Preservation of morphology of the cochlea at a light microscopic level was best with FA or FGA as the fixative and celloidin as the embedding medium.
Immunostaining was successful using all six antibodies in all three fixatives and embedding media. Overall, FA and FGA gave the most the uniform results. There was surprisingly little difference in immunostaining between paraffin and celloidin, while polyester wax was not as good in some cases.
Our results suggest that celloidin may be the embedding medium of choice for both morphologic and pathologic studies including immunostaining.
Acknowledgments
This work was supported by funding from the NIDCD U24 DC0008559. We also thank Mr. Axel Eliasen and Mr. Lakshmi Mittal for support of our work.
References
- Adams JC. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol & Neurotol. 2002;23:316–322. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- Ganbo T, Sando I, Balaban CD, Suzuki C, Sudo M. Immunohistochemistry of lymphocytes and macrophages in human celloidin-embedded temporal bone sections with acute otitis media. Ann Otol Rhinol Laryngol. 1997;106:662–8. doi: 10.1177/000348949710600809. [DOI] [PubMed] [Google Scholar]
- Hafidi A, Despres G, Romand R. Cochlear innervation in the developing rat: an immunocytochemical study of neurofilament and spectrin proteins. J Comp Neurol. 1990;300:153–161. doi: 10.1002/cne.903000202. [DOI] [PubMed] [Google Scholar]
- Hassell J, Hand AR. Tissue fixation with diimidoesters as an alternative to aldehydes. I. Comparison of cross-linking and ultrastructure obtained with dimethylsuberimidate and glutaraldehyde. J Histochem Cytochem. 1974;22:223–239. doi: 10.1177/22.4.223. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Horowitz S, Ruckenstein MJ. Na, K-ATPase in the cochlear lateral wall of human temporal bones with endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1995;104:858–863. doi: 10.1177/000348949510401106. [DOI] [PubMed] [Google Scholar]
- Lopez I, Ishiyama G, Tang Y, Frank M, Baloh RW, Ishiyama A. Estimation of the number of nerve fibers in the human vestibular endorgans using unbiased stereology and immunohistochemistry. J Neurosci Methods. 2005;145:37–46. doi: 10.1016/j.jneumeth.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Luna LG. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. American Histolabs, Inc.; Gaitheresburg, MD: 1992. [Google Scholar]
- Merchant SN, Burgess B, O'Malley J, Jones D, Adams JC. Polyester Wax: A new embedding medium for the histopathologic study of human temporal bones. Laryngoscope. 2006;116:245–249. doi: 10.1097/01.mlg.0000192171.85406.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, Ibrahim S, Gusterson B. Improved immunohistochemical localization of tissue antigens using modified methacarn fixation. J Histochem Cytochem. 1985;33(5):491–495. doi: 10.1177/33.5.3921605. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar architecture in celloidin sections from the human cerebral cortex. J Neurosci Methods. 1999;93(1):69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Portmann D, Fayad J, Wackym PA, Shiroishi H, Linthicum FH, Rask-Andersen H. A technique for reembedding celloidin sections for electron microscopy. Laryngoscope. 1990;100:195–199. doi: 10.1288/00005537-199002000-00017. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Pathology of the ear. ed 2 Lea and Febiger; Philadelphia, Pa: 1993. [Google Scholar]
- Schulte BA, Adams JC. Distribution of immunoreactive Na+, K+-ATPase in gerbil cochlea. J Histochem Cytochem. 1989;37(2):127–34. doi: 10.1177/37.2.2536055. [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. Antigen retrieval techniques: Current Perspectives. J Histochem Cytochem. 2001;49(8):931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- Skepper JN. Immunocytochemical strategies for electron microscopy: choice or compromise. J Microscopy. 2000;199:1–36. doi: 10.1046/j.1365-2818.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- Slepecky NB, Ulfendahl M. Actin-binding and microtubule-associated proteins in the organ of Corti. Hearing Res. 1992;57:201–215. doi: 10.1016/0378-5955(92)90152-d. [DOI] [PubMed] [Google Scholar]
- Stankovic KM, Adams JC, Brown D. Immunolocalization of aquaporin CHIP in the guinea pig inner ear. Am J Physiol. 1995;269:C1450–6. doi: 10.1152/ajpcell.1995.269.6.C1450. [DOI] [PubMed] [Google Scholar]
- Tian Q, Linthicum FH, Keithley EM. Application of labeling techniques to archival temporal bone sections. Ann Otol Rhinol Laryngol. 1999;108:47–53. doi: 10.1177/000348949910800107. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Okada Y. Mechanisms of heat-induced antigen retrieval: analyses in vitro employing SDS-PAGE and immunohistochemistry. J Histochem Cytochem. 2005;53(1):13–21. doi: 10.1177/002215540505300103. [DOI] [PubMed] [Google Scholar]
- Zehnder AF, Adams JC, Santi PA, Kristiansen AG, Wacharasindhu C, Mann S, Kalluri R, Gregory MC, Kashtan CE, Merchant SN. Distribution of type IV collagen in the cochlea in Alport syndrome. Arch Otolaryngol Head Neck Surg. 2005;131:1007–1013. doi: 10.1001/archotol.131.11.1007. [DOI] [PubMed] [Google Scholar]