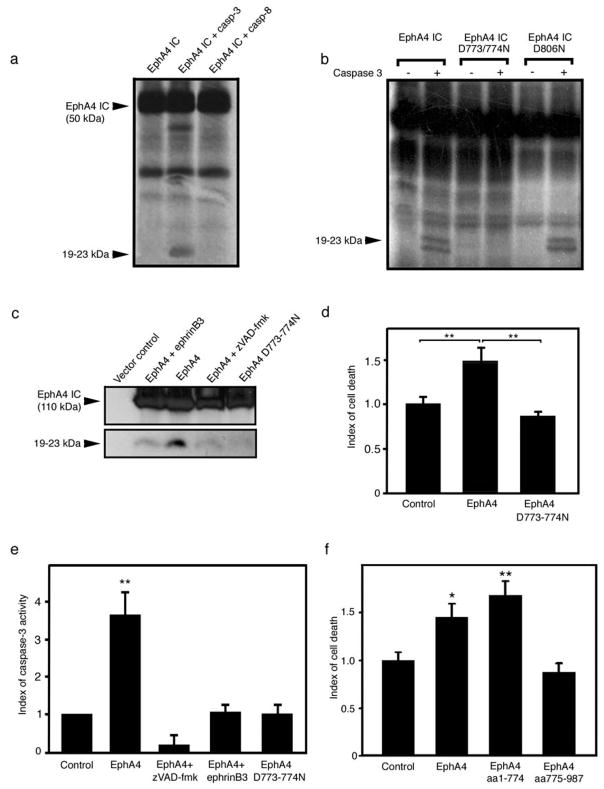
Figure 3.
EphA4 is a substrate for caspase-3 like caspase. (a) The intracellular fragment of EphA4 (aa 571–987) was expressed in vitro and incubated with caspases. Caspase-3, but not caspase-8, triggered the release of smaller fragments (19–23 kDa). The other bands present on the blots most likely reflect aspecific translation initiated at different methionine residues, as shown and described previously [13]. (b) Mutants D>N were generated to locate the caspase cleavage site. Mutation of residues D773/774 prevented the release of the small 19–23 kDa fragments, where lower molecular weight bands were resolved using a gradient gel. The mutant D806N is shown as a control to illustrate the specificity of cleavage. (c) EphrinB3 could block EphA4 cleavage similar to application of zVAD-fmk and the D773/774N mutant EphA4 receptor. (d) The mutant EphA4 D773/774N does not induce significantly greater cell death than the control. (e) The mutant EphA4 D773/774N does not induce greater caspase activation than the control. The levels of caspase activation are similar to the ones observed in the presence of ephrinB3. (f) The pro-apoptotic domain of EphA4 is located upstream from the cleavage site. The two fragments that would be generated upon caspase cleavage (aa1–774 and aa775–987) were over-expressed separately, cell death was observed in the amino terminal fragment but not the carboxy terminal fragment. *p<0.05; **p<0.01.