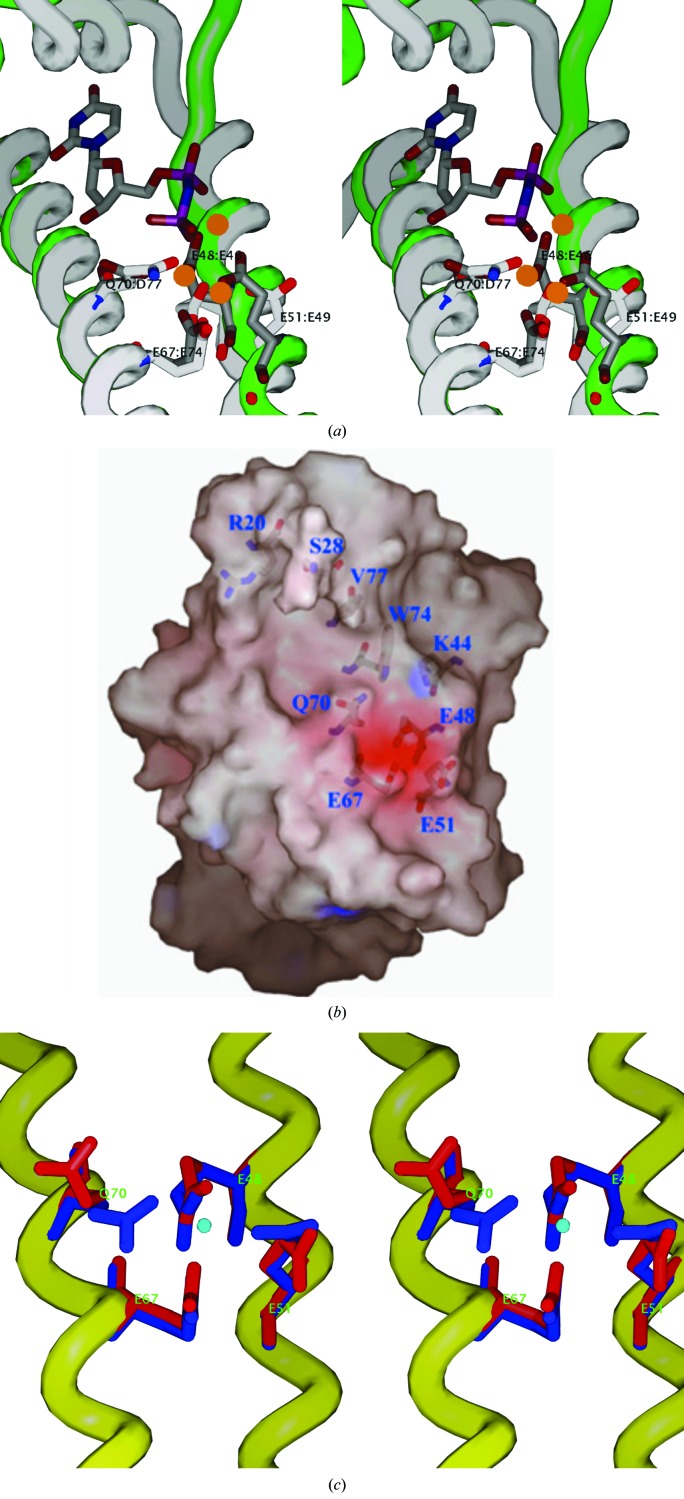
Figure 4.
(a) A superposition of the metal-binding site between Mtb HisE crystal form II (white backbone and side chains) and T. cruzi dUTPase (1w2y; green backbone, gray side chains) complexed with three Mg2+ ions (orange spheres) and a dUDP analog (dUpNHp). The HisE residues (white) are labeled E48, E51, E67 and Q70. The corresponding dUTPase residues (gray) are E46, E49, E74 and D77. This figure illustrates the coordination of all three Mg2+ ions by the side chains in 1w2y (gray), including the central Mg2+ ion, which is coordinated by all four side chains simultaneously, and coordination of two of the Mg2+ ions to the two phosphates in the ligand. Furthermore, the side chains in the HisE structure are in approximately the same conformation, although they appear to be bound only to water molecules, since the HisE structure is uncomplexed. (b) Molecular surface showing the putative substrate-binding site (crystal form I). The substrate-binding pocket is slightly opened (owing to rotation of Gln70 away), but still constitutes a cluster of highly conserved negatively charged residues (E48, E51 and E67). (c) Superposition of the EXXE metal-binding motifs from the form I crystal (red) and form II crystal (blue). The ordered molecule (density peak modeled as a water molecule) in crystal form II is shown in the center (blue sphere) to illustrate the coordination to all four side chains. In contrast, in crystal form I Q70 is flipped away and the carboxylate of E51 is rotated differently.