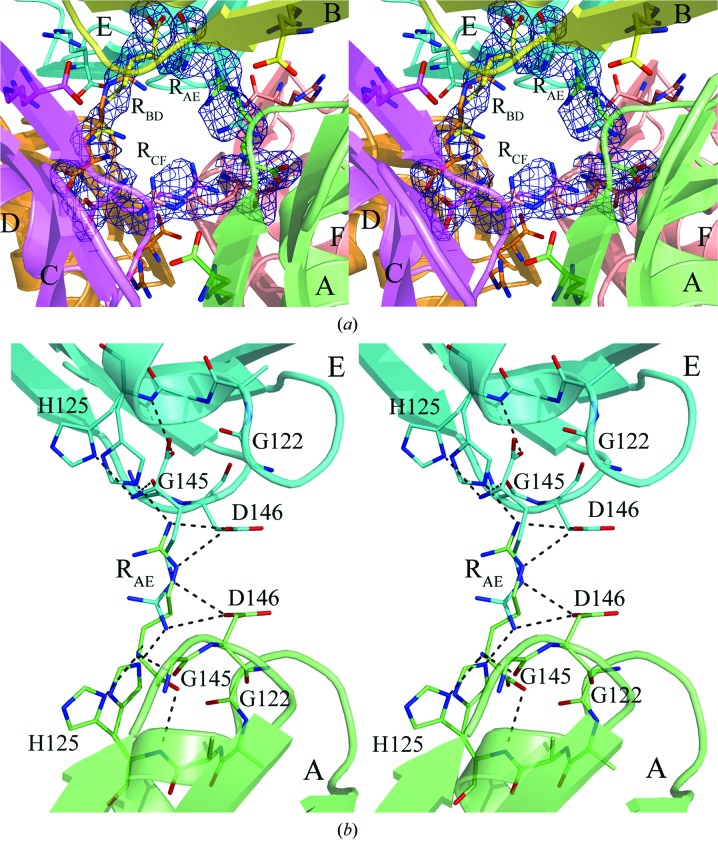
Figure 4.
(a) A close-up of the gap between the trimers of the MtbCArgR hexamer bound with the three additional arginine molecules viewed approximately as in Fig. 3 ▶. The molecule RCF stretches between subunits C and F. The molecules RAE and RBD are stretched between subunits A and E and between subunits B and D, respectively. (b) The binding site for the ligand molecule RAE. Binding residues are represented by sticks. Residue His125 adopts two positions that differ in the orientation of the imidazole group. This group must be located far from the ligand if the latter adopts the position with the α-amino and α-carboxyl groups located near the corresponding His125. The imidazole group of the His125 in the other subunit can adopt both positions in this case. Hydrogen bonds are shown as black broken lines. There are also ion pairs between the ligand guanidinum group and the β-carboxyl groups of the aspartic acid residues Asp146.