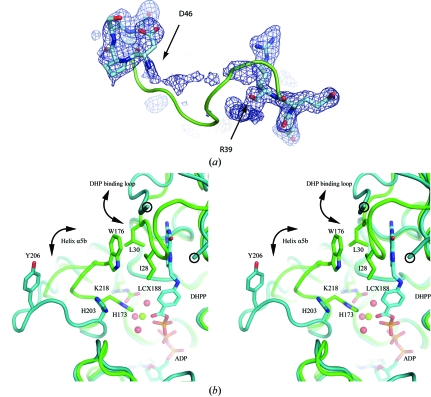
Figure 6.
The DHP-binding loop and its environment. (a) Electron density (from an F o − F c OMIT map contoured at 1.5σ) for the DHP-binding loop of MtFPGS (residues 36–50). No interpretable density is found between Arg39 and Asp46. The positions of these latter residues suggest that the loop has a different conformation from that of EcFPGS, which is shown as a green ribbon. (b) Stereoview showing changes in the DHP-binding loop and the β5–α6 loop in the MtFPGS (teal) and EcFPGS (green) structures. Residues Arg39 and Asp46, which flank the disordered part of the DHP-binding loop in MtFPGS, are circled. The E. coli DHP-binding loop, in contrast, is ordered and is closed over the substrate. DHP binding in EcFPGS leads to the ‘closing’ of the DHP-binding loop from an ‘open’ position similar to that seen in MtFPGS. The ‘closed’ position is stabilized by hydrophobic interactions involving Ile28 and Leu30 from the DHP-binding loop and Trp176 from the β5–α6 loop. In MtFPGS, the open β5–α6 loop causes Tyr206, corresponding to Trp176 in EcFPGS, to move 11 Å away. Other accompanying changes in MtFPGS compared with EcFPGS are the rotation of His203 away from the Mg2-binding site, the loss of this magnesium ion and the noncarbamylation of Lys218. In the EcFPGS structure, phosphorylated DHP (DHPP), Mg2 (green sphere), its water ligands (red spheres) and the carbamylated Lys188, which forms part of the second-shell coordination of Mg2, can all be observed. ADP is situated below the DHPP and is identically positioned in MtFPGS and EcFPGS.