Table 1.
1, 3-Dipolar cycloadditions of TMS diazomethane and various dipolarophiles
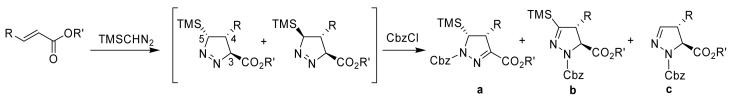 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Cmpd. | R | R’ | Cycloaddition Conditions | %Yielda | Product Ratio a:b:c | Relative configuration of 1-pyrazoline (3, 5 trans: 3, 5-cis) |
| 1 | 5 | H | Me | 2 eq. TMSCHN2, PhCH3 / hexane (1:1), rt, 8.5 hr | 20b | 0:60:40 | Not determined |
| 2 | 6 | H | Et | 1.5 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 24 hr | 90 | 0:35:65 | 50:50 |
| 3 | 7 | H | t-Bu | 1.2 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 24 hr | 71 | 0:44:56 | 50:50 |
| 4 | 8 | H | Mtc | 1.5 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 24 hr | 50 | 0:45:55 | 50:50 |
| 5 | 9 | Me | Et | 3 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8.5 hr | 40 | 0:37:63 | 6:94 |
| 6 | 9 | Me | Et | 3 eq. TMSCHN2, Toluene, reflux, 8.5 hr | 49 | 16:16:68 | 50:50 |
| 7 | 10 | Me | t-Bu | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 47 | 0:76:24 | Not determined |
| 8 | 11 | Me | Mt | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 62 | 0:5050 | Not determined |
| 9 | 12 | Ph | Et | 2 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 8 hr | 7.5 | 5:20:75 | 7:93 |
| 10 | 12 | Ph | Et | 2 eq. TMSCHN2, C6H6/ hexane reflux, 8 hr | - | Complex mixture | Not determined |
| 11 | 13 | Ph | t-Bu | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 35 | 11:33:56 | 10:90 |
| 12 | 13 | Ph | t-Bu | 2 eq. TMSCHN2, Toluene, reflux, 8.5 hr (1:1), reflux, 8 hr | 45 | 43:43:14 | 50:50 |
| 13 | 14 | Ph | Mt | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 71 | 24:0:76 | 100:0 (2-pyrazoline) |
Isolated yield.
Yield based on acryloyl chloride.
Mt = menthyl.
