Abstract
BACKGROUND
Postoperative atrial fibrillation (AF) is a frequent complication following cardiac surgery. Risk factors leading to the development of postoperative AF are not well known and may be influenced by mutations of specific channels involved in atrial repolarization. Recently, the authors have identified three single nucleotide polymorphisms (SNPs) (R87Q, A251T and P307S) in the voltage-gated potassium channel hKv1.5 in a French-Canadian population. Two of these, R87Q and P307S, modified the gating process and the expression level of the hKv1.5 channel.
OBJECTIVES
Considering that these SNPs may accelerate atrial repolarization, it was hypothesized that they may predispose patients to postoperative AF.
METHODS
The authors tested the presence of SNPs in the hKv1.5 channel among 185 patients undergoing coronary artery bypass graft surgery.
RESULTS
In the postoperative period, 96 patients (52%) developed a new onset of AF. A higher prevalence of SNPs was found among patients who developed postoperative AF than in the population without this postoperative arrhythmia (6.25% versus 3.37%; P=0.42). Respective allelic frequencies for R87Q and P307S were 0.52% and 1.56% in the postoperative AF group versus 0% and 0.56% in the non-AF group. Families of the carrier patients were also screened, and several members were found who carried the SNPs but did not have AF. The A251T SNP is not likely to be responsible for AF because it does not modify hKv1.5 channel functions.
CONCLUSIONS
A genetic background that may be involved in the occurrence of postoperative AF was identified. Therefore, R87Q and P307S polymorphisms in hKv1.5, possibly in combination with other risk factors, may influence the development of postoperative AF.
Keywords: hKv1.5, Postoperative atrial fibrillation, Potassium channels, Single nucleotide polymorphism
Abstract
CONTEXTE
La fibrillation auriculaire (FA) postopératoire est une complication fréquente des interventions chirurgicales cardiaques. On n’en connaît pas très bien les facteurs de risque, et il se pourrait que des mutations dans certains canaux ioniques, liés à la repolarisation auriculaire soient mises en cause. Les auteurs ont découvert récemment, dans une population québécoise, trois polymorphismes d’un nucléotide simple (PNS) (R87Q, A251T, P307S) dans le canal hKv1.5. Deux d’entre eux, soit le R87Q et le P307S, ont eu pour effet de modifier le processus d’ouverture et de fermeture du canal hKv1.5 et son degré d’expression.
BUT
Comme ces polymorphismes peuvent accélérer la repolarisation auriculaire, nous avons posé comme hypothèse que ceux-ci pouvaient prédisposer les patients à la FA postopératoire.
MÉTHODE
Les auteurs ont vérifié la présence de polymorphismes dans le canal hKv1.5 chez 185 patients opérés pour un pontage coronarien.
RÉSULTATS
Au cours de la période postopératoire, 96 patients (52 %) ont connu une nouvelle crise de FA. La prévalence de polymorphismes était plus élevée chez ces patients que chez les autres (6,25 % contre 3,37 %; P=0,42). La fréquence allélique du R87Q et du P307S était de 0,52 % et de 1,56 %, respectivement, dans le groupe de sujets ayant fait de la FA postopératoire contre 0,0 % et 0,56 %, respectivement, dans le groupe de sujets n’en ayant pas fait. Les familles des patients concernés ont également fait l’objet de dépistage, et plusieurs membres se sont révélés porteurs de ces PNS mais étaient exempts de FA. Quant au polymorphisme A251T, il n’intervient probablement pas dans l’apparition de la FA parce qu’il ne modifie pas le fonctionnement canal hKv1.5.
CONCLUSIONS
Un facteur génétique susceptible d’être mis en cause dans la FA postopératoire a été découvert. Il se pourrait donc que les polymorphismes R87Q et P307S dans le canal hKv1.5, associés à d’autres facteurs de risque, jouent un rôle dans l’apparition de la FA postopératoire.
With an incidence of 10% to 50% (1), atrial fibrillation (AF) is the most common complication following coronary artery bypass graft (CABG) surgery. AF is associated with a twofold increase in cardiovascular mortality (2) and morbidity (3). Factors predisposing patients to postoperative AF, such as advanced age (4), atrial ischemia (5), intracellular calcium overload (6) and a high level of expression of connexin-40 in atrial myocytes (7), have been reported. In addition, Mandal et al (8) reported an association between anti-heat shock protein 65 antibodies and postoperative AF, suggesting a possible role for antibody-mediated immune response in its pathogenesis.
Moreover, postoperative AF seems to be linked to oxidative stress, and particularly to peroxynitrite production (9). The role of oxidative stress in the incidence of this pathology was confirmed by Carnes et al (10), who demonstrated that ascorbate decreases the incidence of postoperative AF.
Gaudino et al (11) demonstrated a close relationship between the −174G/C interleukin-6 promoter gene variant, the inflammatory response to surgery and the development of postoperative AF. Among 110 patients who underwent CABG, they identified GG, CT and CC genotypes. They also found that the GG genotype was linked to higher postsurgery plasma levels of interleukin-6 and increased postoperative incidence of AF, suggesting a genetic modulation of this pathology (11).
The hKv1.5 gene encodes for the voltage-gated potassium channel hKv1.5, which was identified for the first time in myocytes from the human heart by Fedida et al (12). Recently, we found the heterozygous single nucleotide polymorphisms (SNPs) R87Q, A251T and P307S in this channel in a population of 96 French Canadians (13). An electrophysiological analysis showed that the R87Q and P307S variants diminished the expression level of the channel and slowed its inactivation process in Chinese hamster ovary cells. In addition, the variant R87Q accelerated the rate of hKv1.5 channel opening.
Considering the role of hKv1.5 in human atrial repolarization, and considering that the electrophysiological properties of R87Q and P307S variants may lead to abbreviation of the atrial action potential and effective refractory period durations, we assessed the possibility that these SNPs predispose patients to the development of postoperative AF following cardiac surgery. We screened the hKv1.5 gene of 185 patients who underwent CABG, 96 of whom had postoperative AF, and found a higher but nonsignificant prevalence of the R87Q and P307S SNPs among patients with postoperative AF.
METHODS
Patients enrolled
The present study was carried out on 185 French-Canadian patients who underwent CABG, 96 (52%) of whom developed a new onset of postoperative AF significant enough to require pharmacological treatment (ie, episodes longer than 2 h in duration or recurrent episodes of AF). Exclusion criteria included left ventricular ejection fraction less than 40%, age older than 65 years, chronic obstructive pulmonary disease, documented history of previous AF and perioperative myocardial infarction. The present study was approved by the ethics committee of Laval Hospital (Quebec, Quebec).
DNA preparation
Blood samples of patients were obtained from the GenetICQ tissue bank of the Quebec Heart Institute (Quebec, Quebec), and genomic DNA was extracted from blood using the QIAamp DNA Blood Midi Kit, according to the protocol recommended by the manufacturer (QIAGEN, Canada).
Polymerase chain reaction amplification and sequencing of hKv1.5
Polymerase chain reaction (PCR) primers (Table 1) were initially selected in promoter and coding regions of hKv1.5 using Oligo primer analysis software (version 6.0; MBI, USA), and oligos were synthesized by Medicorp Inc (Canada). PCR amplification was performed on 50 ng of total DNA in a thermal cycler (Peltier Thermal Cycler PTC-200; MJ Research, Canada) with Taq (Promega, USA) and Pfu Turbo (Stratagene, Canada) DNA polymerases according to the manufacturer’s protocol. Samples were denatured for 5 min at 94°C and then cycled by denaturing for 1 min at 94°C, annealing for 1 min at 55°C and extending for 2 min at 72°C (35 cycles). A final 10 min extension period was added.
TABLE 1.
Primers used for polymerase chain reaction amplification and sequencing
| Primer | Nucleotide sequence (5′ to 3′) | Tm (°C)* |
|---|---|---|
| Kv1.5-7F | GGAGGCGGCCAGAATGGGCAGC | 76 |
| Kv1.5-8R | CTGCCGGCTCCTCGTGATCCG | 72 |
| Kv1.5-9F | GTCCTCACCATTGCCCTGCCTG | 72 |
| Kv1.5-10R | CTCCCATTCCCTACTCCACTGC | 70 |
| Kv1.5-12F | TTTGATCGCGCCAGCAAC | 56 |
| Kv1.5-13R | CCGGAGATGTTGATGTGGAC | 62 |
| Kv1.5-14F | AGGAGGAAGAAGGCGATCC | 60 |
| Kv1.5-17R | AAGATGGCCACCACATCGAT | 60 |
| Kv1.5-18F | AGCTGCTCGTGCGCTTCTT | 60 |
| Kv1.5-19R | GCTCCTCGTGATCCGTTTC | 60 |
Temperature (Tm) = 4(G+C)+2(A+T)
PCR products were visualized and purified on an agarose gel stained with ethidium bromide and sequenced on both strands. Nucleotide sequence was determined at the Nucleic Acids Analysis and Synthesis Service of Laval University (Quebec, Quebec) on an Applied Biosystem 3100 genetic analyzer (Perkin-Elmer, Canada) using the fluorescent dideoxy terminator sequencing protocol as recommended by the manufacturer. Electrophoretograms were visualized using Chromas software (version 1.45; Griffith University, Australia). Sequence comparisons between the wild-type and the hKv1.5 gene of affected subjects were performed using ClustalW (EMBL-EBI, United Kingdom).
Statistical analysis
To evaluate whether the SNPs R87Q, A251T and P307S were significantly linked to post-CABG AF, χ2 analysis was used. The 96 patients with post-CABG AF were compared with the 89 post-CABG patients without AF and with a control population of 95 Caucasian patients (14). P<0.05 was considered to be significant. Values are presented as mean ± SD.
RESULTS
Clinical characteristics of patient groups
There was no significant difference in the mean age (55±6 years for non-AF and 58±5 years for AF) or the sex distribution (77.5% men in the non-AF group versus 86.5% in the AF group) of both groups. Moreover, no difference was found between mean left ventricular ejection fraction (64±12% in the non-AF group versus 64±13% in the AF group).
Molecular analysis of hKv1.5 in patients
An increased incidence of SNPs was documented in the post-CABG AF group compared with the group without postoperative AF (6.25% versus 3.37%; P=0.42). Among the patients with postoperative AF, one patient was identified with the R87Q SNP and two patients were identified with the A251T SNP, whereas the P307S SNP was found in three other patients. In the post-CABG group without AF, only A251T and P307S were found in two patients and one patient, respectively. All patients were heterozygous for these SNPs; their respective allelic frequencies are presented in Table 2. Although the results are not statistically significant, when studied individually, the allelic frequencies of the R87Q and P307S SNPs were higher in the post-CABG group with AF (0.52% and 1.56%, respectively, versus 0% and 0.56% in the non-AF group). The allelic frequencies were also compared between the AF group and the 95 control patients without AF, who were recently screened for hKv1.5 by Simard et al (14). A251T and P307S SNPs were identified in this population, with allelic frequencies of 0.53% for both (14). P values for the frequency of occurrence of R87Q, A251T and P307S in the post-CABG AF group compared with the non-AF group and this control population were 1.00, 0.87 and 0.63, respectively.
TABLE 2.
Distribution of the R87Q, A251T and P307S single nucleotide polymorphisms of hKv1.5 among a group of post-coronary artery bypass graft (CABG) surgery patients with and without atrial fibrillation (AF), and in a control Caucasian population
| Variant | Post-CABG AF (n=96) | Post-CABG without AF (n=89) | Caucasian population (n=95)* | P |
|---|---|---|---|---|
| R87Q | 1 (0.52%) | 0 (0.00%) | 0 (0.00%) | 1.00 |
| A251T | 2 (1.04%) | 2 (1.12%) | 1 (0.53%) | 0.87 |
| P307S | 3 (1.56%) | 1 (0.56%) | 1 (0.53%) | 0.63 |
The number of patients carrying single nucleotide polymorphisms and the allelic frequencies are indicated in each group.
Data from Simard et al (14)
Molecular analysis of hKv1.5 in family members
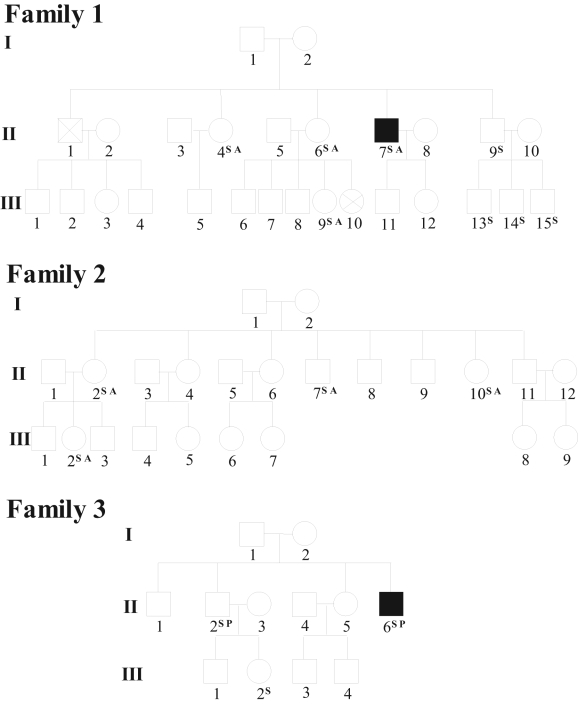
The presence of SNPs was tested in family members of carrier patients. Twelve members in three families were enrolled in the study and were screened for hKv1.5. The pedigrees of the three families are shown in Figure 1. In family 1, the A251T SNP was present in a post-CABG patient with AF (II.7 in Figure 1) and in three of the seven members who took part in the study. In family 2, the same SNP was found in a post-CABG patient without AF (II.7) and in all three members enrolled. Finally, P307S was identified in one member of the family of a post-CABG patient with AF (II.6 of family 3). All family members were heterozygous for these SNPs. Because none of the family members for whom SNPs were screened had presented with any documented episodes of AF, the presence of other factors may be necessary to trigger this arrhythmia.
Figure 1.
Pedigrees of the families of three post-coronary artery bypass graft (CABG) surgery patients carrying single nucleotide polymorphisms in hKv1.5. Squares represent men, circles represent women and crosses represent deceased persons. Solid symbols represent patients with post-CABG atrial fibrillation; in family 2, the proband II.7 represents a post-CABG patient without atrial fibrillation. A A251T; P P307S; S Family members for whom the hKv1.5 gene was sequenced
DISCUSSION
We performed a molecular analysis of hKv1.5 in 185 French Canadians who underwent CABG, 96 of whom had postoperative episodes of AF. We have previously characterized, in a French-Canadian population, the R87Q, A251T and P307S SNPs in this gene (13). These variants were found among post-CABG patients with AF, with respective allelic frequencies of 0.52%, 1.04% and 1.56%. In the group without AF, the R87Q SNP was not identified, but the two other variants were encountered (allelic frequencies of 1.12% and 0.56% for A251T and P307S, respectively). Overall, we found a higher prevalence of SNPs among patients with postoperative AF, suggesting that genetic background may, with other concomitant factors related to surgery, predispose patients to the development of postoperative AF. Even though the sample size was not sufficient to establish statistical significance for each individual mutation, the results suggest that these mutations may influence the development of postoperative AF. Indeed, these SNPs slow the inactivation process of the hKv1.5 current and, in addition, R87Q increases the rate of channel opening (13). Thus, these mutations accelerate atrial repolarization, which contributes to action potential shortening during AF.
A nonsense mutation in hKv1.5 (E375X) has recently been associated with one familial case of AF (15), which demonstrates the pertinence of analyzing this potassium channel in patients suffering from AF. In addition to the hKv1.5 gene, to date, only a few other genes, KvLQT1 (16,17), Kir2.1 (18) and MiRP1 (19), have been found to be associated with AF in a very limited number of patients. Of note, all these genes encode for subunits of cardiac potassium channels, and all the identified mutations potentially shorten the atrial action potential duration and effective refractory period. The effects of R87Q and P307S on hKv1.5 channel properties suggest that these SNPs may be involved in the familial and the postoperative forms of AF. The A251T variant is not likely to be responsible for AF because it does not modify the electrophysiological properties of hKv1.5 (13). R87Q and P307S SNPs may not promote AF by themselves, but they may be implicated in the development of this disease, in combination with other factors, such as the oxidative stress related to surgery. Indeed, epidemiological studies have shown that genetic and environmental factors can be implicated in the development of disease and that none of these isolated factors can independently promote the pathology (20,21). It has been shown that postoperative AF is associated with oxidative stress, and particularly with peroxynitrite formation (9,10). A recent study (22) has also shown that hydrogen peroxide increases the hKv1.5 current amplitude at voltages corresponding to the action potential repolarization phase and accelerate hKv1.5 channel opening. Thus, the presence of R87Q and P307S SNPs, in conjunction with other stress factors associated with surgery such as a burst of oxygen free radical release, may trigger postsurgery AF.
Several studies suggest that up to one-third of all patients with AF have no obvious cause and are referred to as having lone AF (23,24). The absence of other causes associated with a high incidence of family history suggest the presence of a monogenic disease. As pointed out by Roberts (25), although seven chromosomal loci mapped and four genes identified so far have been found in families with lone AF, the small size of investigated families has precluded identification of genes by conventional genetic linkage analysis. Other case-control association studies in patients with structural heart disease and AF suggested a predisposition to AF in patients with a polymorphism in the angiotensin-converting enzyme gene (26) or the connexin-40 gene (27). However, the number of patients in these studies was insufficient, and additional analyses are needed before making a conclusion. As in all polymorphism studies previously reported, our study was observational, and a higher patient population sample is clearly needed. The sample size may need to be 1000 or more patients and will certainly need a multicentre approach. However, we believe that our findings merit to be reported as a pilot study to consider the feasibility of assessing the relationships between polymorphisms in hKv1.5 and AF in patients following CABG surgery.
CONCLUSIONS
The frequencies of SNPs were elevated in patients who developed postoperative AF following CABG surgery. Thus, we propose that a genetic background associated with surgery-related factors, which are poorly understood at the moment, predispose patients to the development of a new onset of postoperative AF. Although the problem of AF has been tackled by many researchers, it still remains unclear why some patients, even when they have no apparent risk factors, develop postoperative AF, while others do not. Given the effects of R87Q and P307S on the electrophysiological properties of the hKv1.5 channel, these SNPs may be linked, when combined with other factors, to postoperative AF. However, it is necessary to screen the hKv1.5 gene of one thousand or more patients to establish this hypothesis. Such a study may be helpful in providing better insight into the role of the genetic basis of AF in the postoperative period and, ultimately, in limiting the incidence of postoperative AF by improved and/or preventive treatment.
ACKNOWLEDGEMENTS
This work was supported by the Canadian Institutes of Health Research (CIHR-MOP 64383), the Quebec Heart Institute, and the Heart and Stroke Foundation of Canada (Dr Pascal Daleau). Dr Isabelle Plante was the recipient of a CIHR-Rx&D PhD studentship. The authors thank Dr Laimonis Gailis for reviewing the manuscript. Dr Patrick Mathieu is a research scholar of the Fonds de la Recherche en Santé du Québec, Montreal, Quebec.
REFERENCES
- 1.Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989;97:821–5. [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 4.Lévy S. Epidemiology and classification of atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9:S78–82. [PubMed] [Google Scholar]
- 5.Kolvekar S, D’Souza A, Akhtar P, Reek C, Garratt C, Spyt T. Role of atrial ischaemia in development of atrial fibrillation following coronary artery bypass surgery. Eur J Cardiothorac Surg. 1997;11:70–5. doi: 10.1016/s1010-7940(96)01095-0. [DOI] [PubMed] [Google Scholar]
- 6.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–36. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 7.Dupont E, Ko Y, Rothery S, et al. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation. 2001;103:842–9. doi: 10.1161/01.cir.103.6.842. [DOI] [PubMed] [Google Scholar]
- 8.Mandal K, Jahangiri M, Mukhin M, Poloniecki J, Camm AJ, Xu Q. Association of anti-heat shock protein 65 antibodies with development of postoperative atrial fibrillation. Circulation. 2004;110:2588–90. doi: 10.1161/01.CIR.0000136825.96029.A5. [DOI] [PubMed] [Google Scholar]
- 9.Mihm MJ, Yu F, Carnes CA, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 10.Carnes CA, Chung MK, Nakayama T, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 11.Gaudino M, Andreotti F, Zamparelli R, et al. The –174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(Suppl 1):II195–9. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 12.Fedida D, Wible B, Wang Z, et al. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res. 1993;73:210–6. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- 13.Plante I, Fournier D, Ricard G, et al. Electrophysiological characterization of three non-synonymous single nucleotide polymorphisms (R87Q, A251T, and P307S) found in hKv1.5. Pflugers Arch. 2006;452:316–23. doi: 10.1007/s00424-005-0031-8. [DOI] [PubMed] [Google Scholar]
- 14.Simard C, Drolet B, Yang P, Kim RB, Roden DM. Polymorphism screening in the cardiac K+ channel gene KCNA5. Clin Pharmacol Ther. 2005;77:138–44. doi: 10.1016/j.clpt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Olson TM, Alekseev AE, Liu XK, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–91. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 16.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–4. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 17.Hong K, Piper DR, Diaz-Valdecantos A, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68:433–40. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Xia M, Jin Q, Bendahhou S, et al. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–9. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Xia M, Jin Q, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenbroucke JP, Koster T, Briët E, Reitsma PH, Bertina RM, Rosendaal FR. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet. 1994;344:1453–7. doi: 10.1016/s0140-6736(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 21.van Asselt KM, Kok HS, van der Schouw YT, Peeters PH, Pearson PL, Grobbee DE. Role of genetic analyses in cardiology: Part II: Heritability estimation for gene searching in multifactorial diseases. Circulation. 2006;113:1136–9. doi: 10.1161/CIRCULATIONAHA.105.563197. [DOI] [PubMed] [Google Scholar]
- 22.Caouette D, Dongmo C, Bérubé J, Fournier D, Daleau P. Hydrogen peroxide modulates the Kv1.5 channel expressed in a mammalian cell line. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:479–86. doi: 10.1007/s00210-003-0834-0. [DOI] [PubMed] [Google Scholar]
- 23.Kopecky SL, Gersh BJ, McGoon MD, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–74. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 24.Darbar D, Herron KJ, Ballew JD, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–92. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 25.Roberts R. Mechanisms of disease: Genetic mechanisms of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2006;3:276–82. doi: 10.1038/ncpcardio0509. [DOI] [PubMed] [Google Scholar]
- 26.Tsai CT, Lai LP, Lin JL, et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109:1640–6. doi: 10.1161/01.CIR.0000124487.36586.26. [DOI] [PubMed] [Google Scholar]
- 27.Firouzi M, Ramanna H, Kok B, et al. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95:e29–33. doi: 10.1161/01.RES.0000141134.64811.0a. [DOI] [PubMed] [Google Scholar]