Table 2.
Preparation of 3-Sulfenyl- and 3-Selenylindoles by n-Bu4NI-Induced Electrophilic Cyclizationa
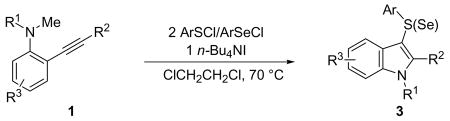 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 1 | R1 | R2 | R3 | ArSCl/ArSeCl | time (h) | 3 | % yieldb |
| 1 | 1a | Me | C6H5 | H | p-O2NC6 H5SCl | 5 | 3a | 90 |
| 2 | 1a | Me | C6H5 | H | F5C6 SCl | 5 | 3b | 87 |
| 3 | 1a | Me | C6H5 | H | C6H5 SCl | 6 | 3c | 87 |
| 4 | 1a | Me | C6H5 | H | p-MeC6 H5SCl | 6 | 3d | 92 |
| 5 | 1a | Me | C6H5 | H | o-O2NC6 H5SCl | 5 | 3e | 52 |
| 6 | 1a | Me | C6H5 | H | C6H5SeCl | 5 | 3f | 84 |
| 7 | 1b | Me | C6H5 | 6-Me | p-O2NC6 H5SCl | 9 | 3g | 78 |
| 8 | 1c | Me | C6H5 | 5-Br | p-O2NC6 H5SCl | 6 | 3h | 85 |
| 9 | 1d | Me | C6H5 | 5-CO2Me | C6H5 SCl | 8 | 3i | 75 |
| 10 | 1e | Me |
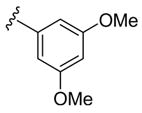
|
H | p-O2NC6 H5SCl | 9 | 3j | 74 |
| 11 | 1f | Me |
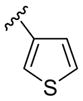
|
H | p-O2NC6 H5SCl | 3 | 3k | 85 |
| 12 | 1g | Me |
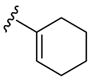
|
H | p-O2NC6 H5SCl | 3 | 3l | 91 |
| 13 | 1h | Me |
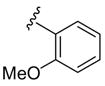
|
H | C6H5 SCl | 4 | 3m | 79 |
| 14 | 1i | C6H5 |
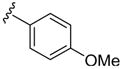
|
H | p-MeC6 H5SCl | 3 | 3n | 99 |
Representative procedure: N,N-dialkyl-2-(1-alkynyl)aniline1 (0.50 mmol), n-Bu4NI (0.50 mmol), arylsulfenyl/arylselenenyl chloride (1.00 mmol), and 5 mL of DCE were mixed in a sealed 4-dram vial. The reaction was stirred at 70 °C for the indicated time.
Isolated yields after column chromatography.
