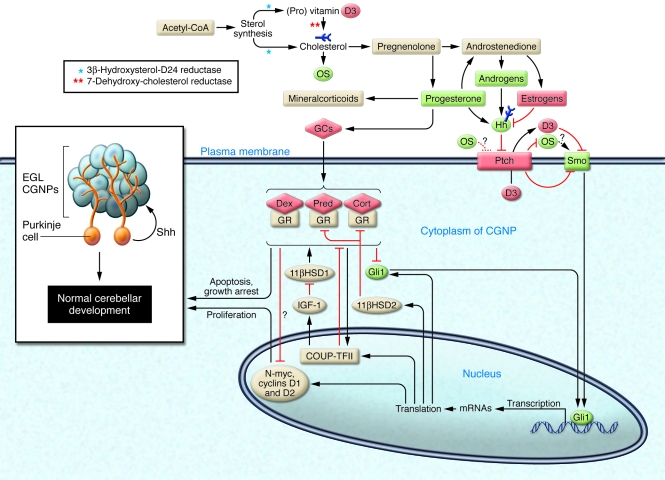
Figure 1. Crosstalk between Hh and steroid hormones during neuronal development and injury.
Steroid hormone synthesis starts with acetyl-CoA and leads to the formation of (pro) vitamin D3 and cholesterol. Cholesterol-derived pregnenolone is synthesized from and is the origin of all steroid hormones. Cholesterol also gives rise to oxysterols (OS), important positive mediators of Hh pathway activation. Inhibition or mutation of enzymes involved at early steps of steroid hormone biosynthesis (asterisks) blocks cholesterol and (pro) vitamin D3 production, leading to defects in Hh signaling. Progesterone induces Ihh in the uterus, androgens upregulate Shh in the developing prostate, and estrogens downregulate Shh in the prostate and Ihh and Dhh in the uterus. In this issue of the JCI, Heine and Rowitch report that GCs can modulate the Hh pathway in postnatal mice (10). Proliferation of CGNPs in the cerebellar external granule layer (EGL) is physiologically promoted by Shh secreted by Purkinje cells. This process is antagonized by binding of GCs to the GR, which also leads to CGNP apoptosis. GC antagonism of Hh signaling leads to neuronal injury and aberrant cerebellar development. Shh protects CGNPs via 11βHSD2, which inactivates cortisol, Cort, and Pred, but not Dex, suggesting that it is better to use 11βHSD2-sensitive GCs to avoid neuronal injury in neonates. GCs may exert their effects via interaction with COUP-TFII: the GC-bound GR stimulates COUP-TFII–induced transactivation, while COUP-TFII represses GR transcriptional activity (27). COUP-TFII is also an Hh target and modulates the expression of IGF-1, which regulates GC metabolism through 11βHSD1. Positive and negative regulators of the Hh pathway are shown in green and red, respectively.