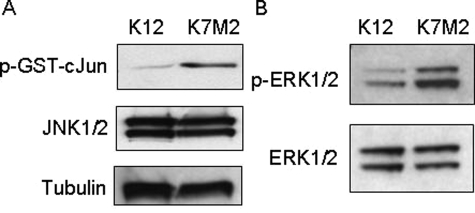
Figure 4.
JNK and MEK1/2 kinase activity is increased in K7M2 cells. A: JNK assays were performed using protein extracted in a nondenaturing lysis buffer. Equal amounts of protein from K12 and K7M2 cells were incubated with a GST-cJun fusion protein linked to agarose beads to pull down JNK in the extracts. In vitro kinase reactions were performed in the presence of cold ATP and phosphorylation of the GST-cJun fusion protein at Ser63 were detected using a phospho-specific antibody in Western blot analysis. JNK1/2 and β-tubulin levels are shown, indicating that equal amounts of proteins were used in the assay. B: MEK1/2 activity was assayed by determining the phosphorylation status of ERK1/2. Equal amounts of K12 and K7M2 protein were used in Western blot analysis and p-ERK1/2 and total ERK1/2 levels are shown. The results shown are representative of three independent experiments.