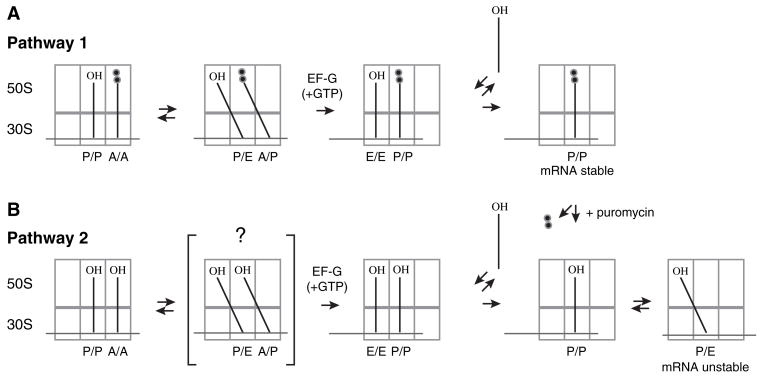
Figure 2. The Destabilization of mRNA Observed Is Hypothesized to Result from Movement of tRNA into the P/E Hybrid State.
Translocation of tRNA within the ribosome is believed to occur in a step-wise fashion (Pathway 1). After peptide bond formation, the newly deacylated tRNA and newly formed peptidyl-tRNA move first with respect to the 50S subunit into the hybrid P/E and A/P states, respectively. EF-G (+GTP) then catalyzes the movement of tRNA and mRNA with respect to the 30S subunit. Because interaction of tRNA in the E/E state is kinetically labile (Robertson and Wintermeyer, 1987; Semenkov et al., 1996), peptidyl-tRNA bound to the P/P state is presumably sufficient to retain stable mRNA interaction in this posttranslocation complex. EF-G(+GTP) can also catalyze the translocation of deacylated tRNA within the ribosome (Pathway 2), although it is unclear whether this reaction involves the hybrid state intermediate. In this posttranslocation complex, tRNA can occupy either the P/P or the P/E state. It is hypothesized that movement of tRNA into the P/E state destabilizes mRNA in this posttranslocation complex, which results in slippage of mRNA. Deacylation of peptidyl-tRNA in the posttranslocation complex of Pathway 1 by addition of puromycin also allows movement of tRNA into the P/E state, and destabilization of mRNA is also observed in this case (see Figure 1).