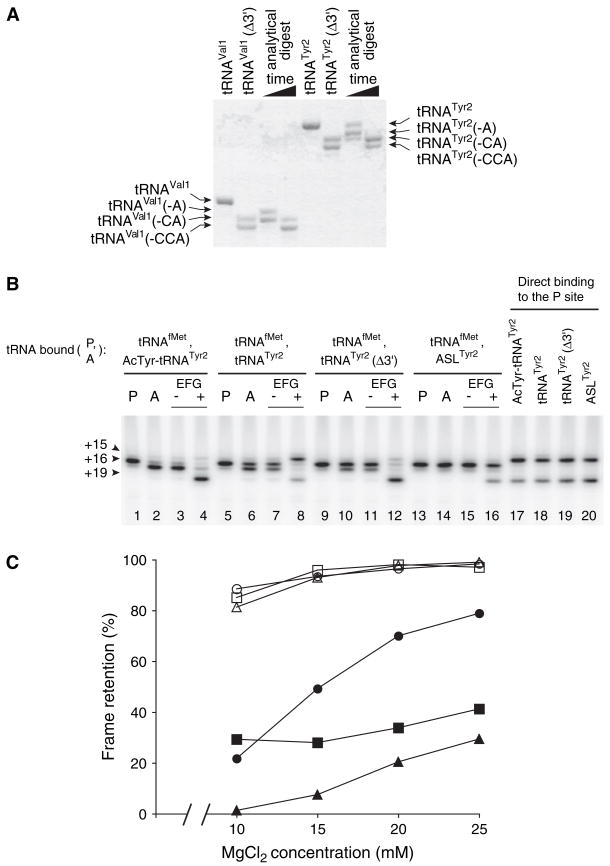
Figure 3. Slippage of mRNA Conferred by Translocation of Deacylated tRNA Is Suppressed by Its 3′ Truncation.
(A) Preparations of 3′-truncated tRNAs (tRNAVal1Δ3′ and tRNATyr2Δ3′ ) were made by limited digestion with snake venom phosphodiesterase. Denaturing PAGE and methylene blue staining indicates that in each of these preparations, ~60% of the molecules lack nucleotides C74–A76 and ~40% lack C75–A76. Analytical digests were loaded in adjacent lanes to provide size markers.
(B) Toeprinting was used to map the position of mRNA within the ribosome before and after EF-G-dependent translocation of several forms of tRNATyr2. Pretranslocation complexes were made by incubating ribosomes with m299 and tRNAfMet to fill the P site (P lanes) and then adding N-acetyl-Tyr-tRNATyr2, tRNATyr2, tRNATyr2Δ3′, or ASLTyr2 to bind the A site (− lanes) as indicated. Complexes were then incubated in the presence of GTP alone (2 lanes) or EF-G plus GTP (+ lanes). In parallel, mRNA was mapped in complexes formed by binding N-acetyl-Tyr-tRNATyr2, tRNATyr2, tRNATyr2Δ3′, or ASLTyr2 directly to the P site (lanes 17–20).
(C) Slippage of mRNA conferred by the translocation of deacylated tRNA is partially suppressed by increasing the concentration of MgCl2 in the translocation reaction. Pretranslocation complexes containing either N-acetyl-aminoacyl-tRNA (open symbols) or deacylated tRNA (closed symbols) bound to the A site were mixed into translocation reactions containing EF-G and GTP such that the final concentration of MgCl2 differed as indicated. Reading-frame retention was determined after translocation of m291 with N-acetyl-Val-tRNAVal1 (○) or tRNAVal1 (●), m299 with N-acetyl-Tyr-tRNATyr2 (□) or tRNATyr2 (■), and m301 (Fredrick and Noller, 2003) with N-acetyl-Phe-tRNAPhe (△) or tRNAPhe (▲).