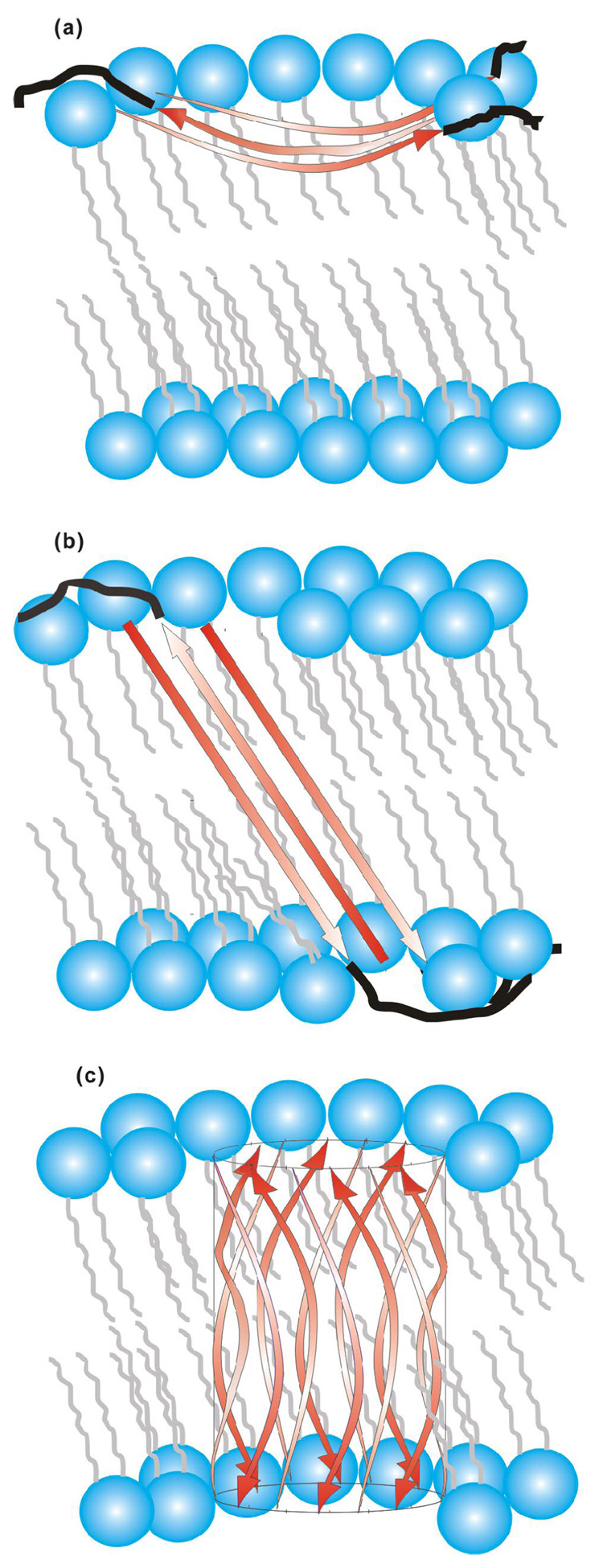
Figure 8.
(a) Partial membrane insertion (PMI) and (b, c) full membrane insertion (FMI) models for antiparallel β strand HFP. The red arrows represent the A1 to G16 residues in strand conformation and the black lines represent the S17 to S23 residues in random coil conformations. For clarity, black lines are not displayed in c. Lipids are represented in blue and grey and cholesterol is not displayed. Three antiparallel strands are displayed in a, b and twelve strands are displayed in c but the actual number of strands in the oligomer/aggregate is not known. The curvature and angle of the strands with respect to the bilayer normal are not known but the models consider that A1-G16 has ~55 Å length and that the transbilayer distance is ~48 Å (100). The experiments do not provide information about the membrane locations of residues S17 to S23. relative to FMI model (b), the FMI β barrel variant (c) could have reduced energy because all of the residues in the membrane interior have backbone hydrogen bonds.