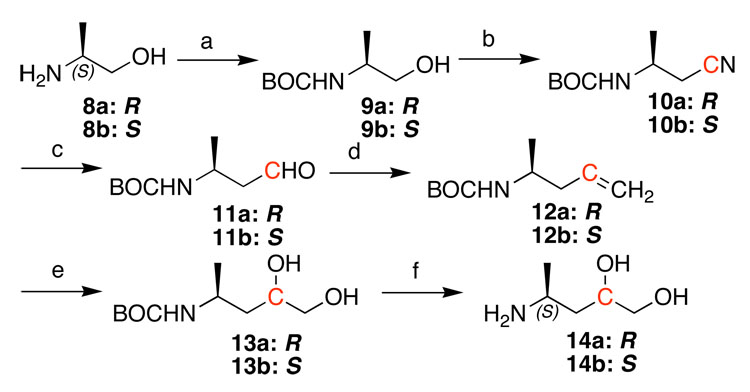
Scheme 2.
Preparation the 13C-labeled amino diols used for site-specific synthesis of adducts 2a and 2b in oligodeoxynucleotides. The S diastereomer is shown. Reagents: (a) (Boc)2O, 1 M NaOH, overnight, 81.5%, (b) MsCl, Et3N, CH2Cl2, rt, 2 hr; K13CN, DMSO, 40 °C, 15 hr, 69% over 2 steps, (c) DIBAH, CH2Cl2, −78 °C, 32%, (d) Me3PCH2Cl,t-BuOK, THF, 70%, (e) OsO4, NMP, THF/t-BuOH/H2O, 76%, (f) Amberlist-H, CH2Cl2/CH3OH; 4 M NH3 in CH3OH, 91%. The antipodal 4R-enantiomer 14a was prepared by an identical sequence starting from commercially available (R)-2-amino-1-propanol.