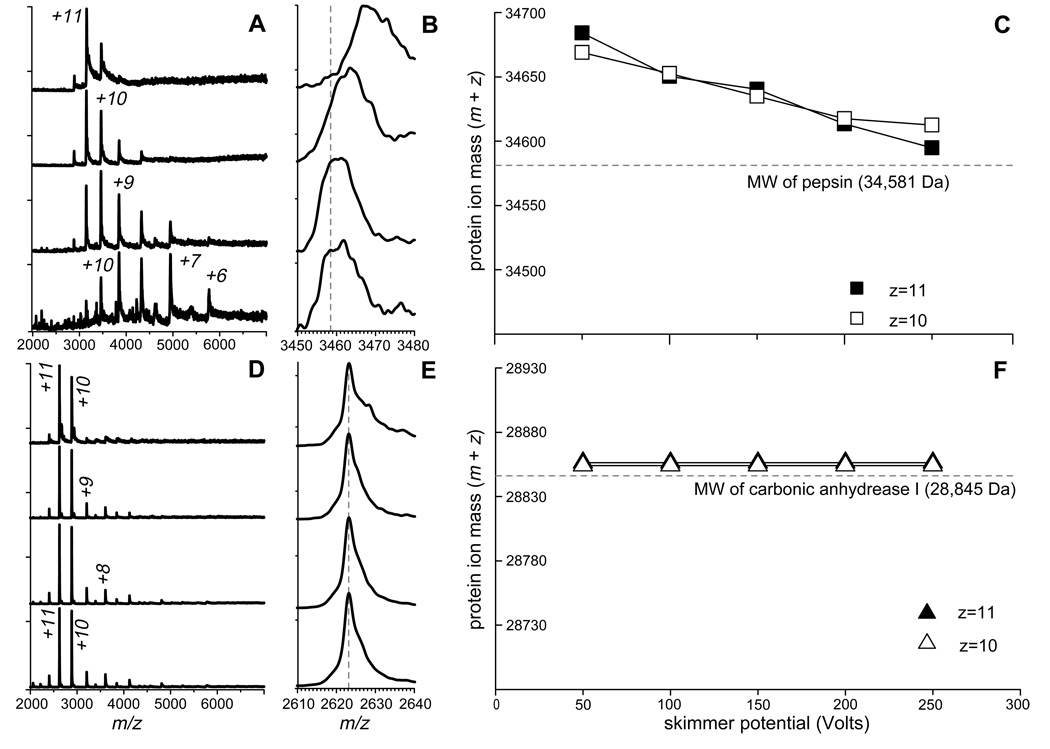
Figure 5.
ESI MS of pepsin (A–C) and carbonic anhydrase I (D–F) acquired at pH 5 (pepsin) and 7 (CA I). Panels (A) and (D) show evolution of protein ion charge state distributions as a function of increasing de-clustering potential from top to bottom in 50 V increments. Panels (B) and (E) show evolution of protein ion peak shapes under the same conditions. Dotted lines represent masses of multiply protonated proteins, which are calculated based solely on their molecular weights and the requisite number of protons. Protein ion mass change as a function of de-clustering potential is shown in panels (C) and (F) for the most abundant ionic species.