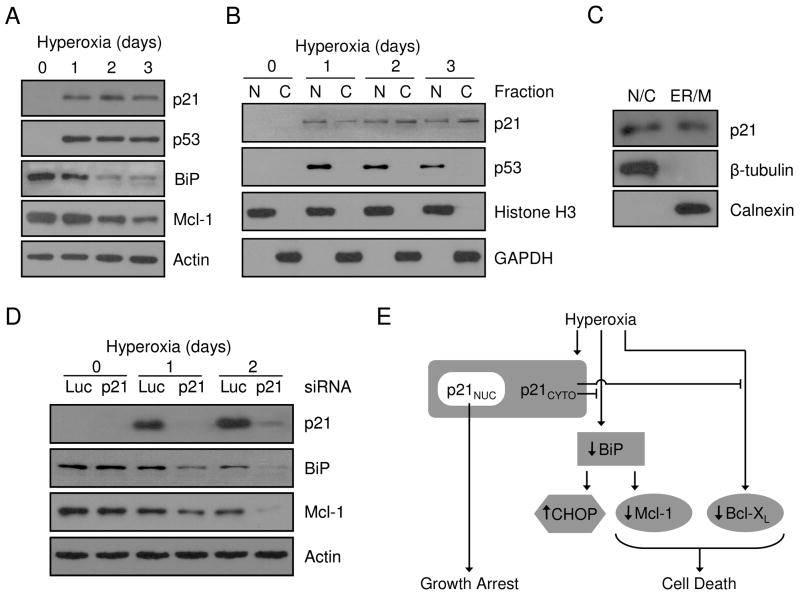
Figure 8. Endogenous p21 localizes to the cytoplasm and regulates BiP and Mcl-1 during hyperoxia.
A549 cells were cultured for 0, 1, 2 and 3 days in hyperoxia and (A) protein was isolated from total cellular lysate or (B) cells were fractionated into nuclear (N) and cytoplasmic (C) compartments prior to protein isolation. Total proteins were immunoblotted for p21, p53, BiP and actin was used as a loading control. Nuclear and cytoplasmic proteins were blotted for p21, p53, Histone H3 and GAPDH. (C) Proteins were isolated from nuclear/cytosolic (N/C) and ER/mitochondria (ER/M) fractions from A549 cells cultured in hyperoxia for 2 days and immunoblotted for p21, β-tubulin and calnexin. (D) A549 cells were transfected with 100 nM siRNA oligos targeting luciferase (Luc) or p21 for 24 hrs and exposed to hyperoxia for 0, 1 and 2 days. Isolated protein was blotted for p21, BiP and Mcl-1 expression and actin was used as a loading control. All immunoblots are representative of at least three separate experiments with similar results. (E) Schematic summary of p21 protective pathways during hyperoxic stress.