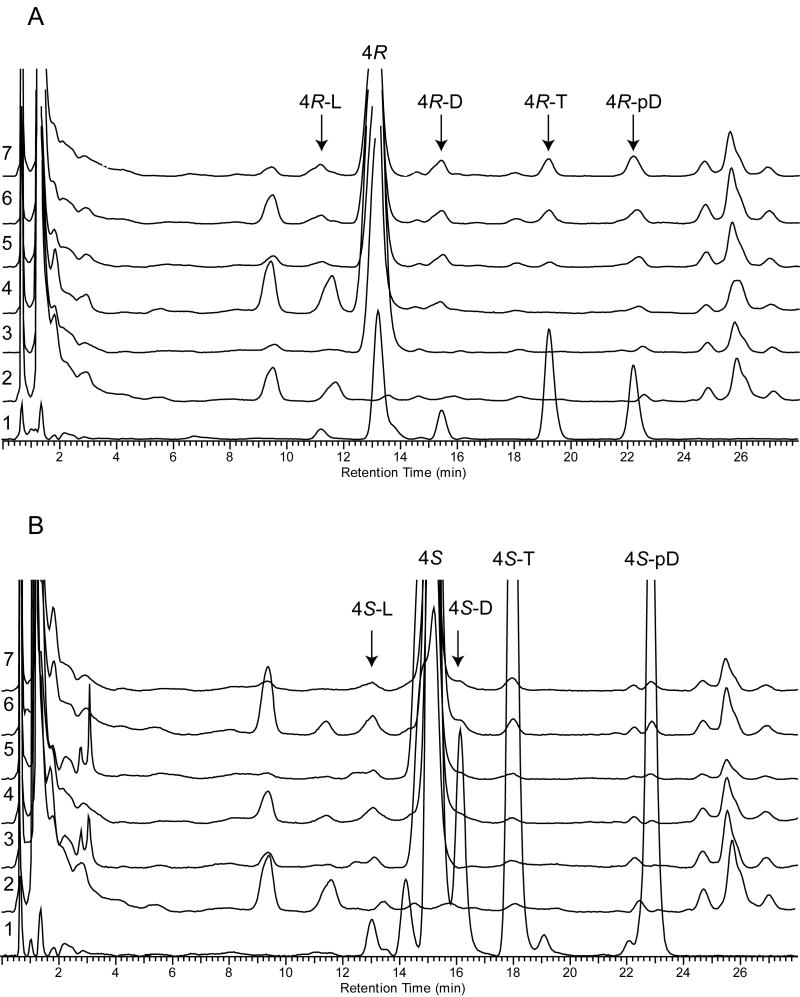
Figure 5. Ex vivo production of pregna-5,7-diene-3β,17α,20-triols (4R and 4S) derivatives in human neonatal skin.
Skin fragments were treated with 2μL of 10 mM of 4R (Panel A) or 4S (Panel B) and subjected to UV irradiation. The products of photolysis in skin were subjected to RP-HPLC chromatography (chromatograms 2-7) and their retention times were compared with previously identified products of UV irradiation of 4R and 4S in methanol (chromatogram 1). Different UV doses were used to show dynamics of the process. The UVB doses per cm2 were as follows: chromatograms 1 and 7 – 2500 mJ/cm2, 3 – 0 mJ/cm2, 4 – 100 mJ/cm2, 5 – 500 mJ/cm2, 6 – 1000 mJ/cm2. Chromatograms 2 show normal profile of non-treated and non-irradiated skin.