Table 1.
Azomethine ylide generation from vinylogous amides via sequential O-sulfonylation and nucleophilic exchange.
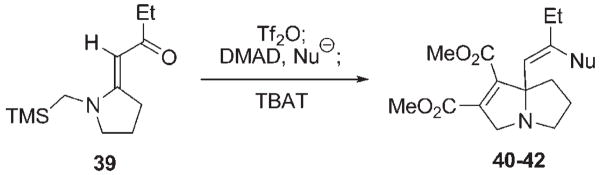 | |||
|---|---|---|---|
| Entry | Bu4N+Nu− | Cycloadduct | Yield [%] |
| 1 | Bu4NI | 40 (Nu=I) | 52 |
| 2 | Bu4NBr | 41 (Nu=Br) | 45 |
| 3 | Bu4NCl | 42 (Nu=Cl) | 52 |
Azomethine ylide generation from vinylogous amides via sequential O-sulfonylation and nucleophilic exchange.
 | |||
|---|---|---|---|
| Entry | Bu4N+Nu− | Cycloadduct | Yield [%] |
| 1 | Bu4NI | 40 (Nu=I) | 52 |
| 2 | Bu4NBr | 41 (Nu=Br) | 45 |
| 3 | Bu4NCl | 42 (Nu=Cl) | 52 |