Abstract
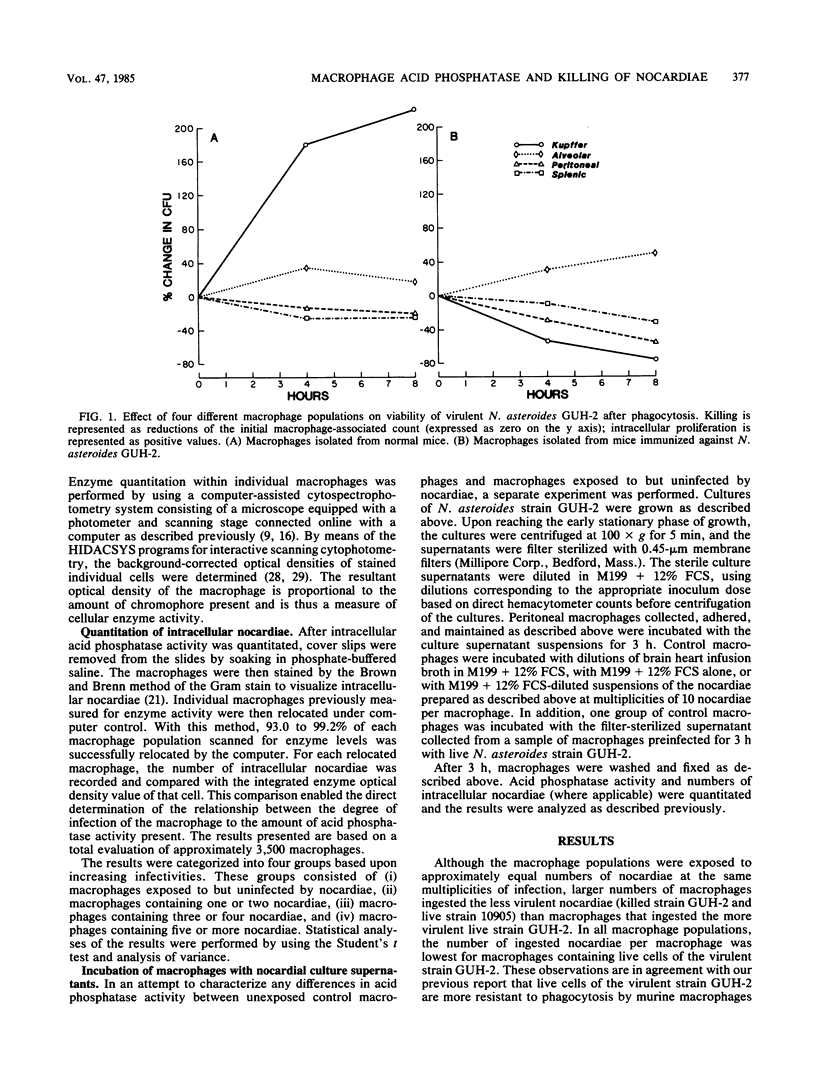
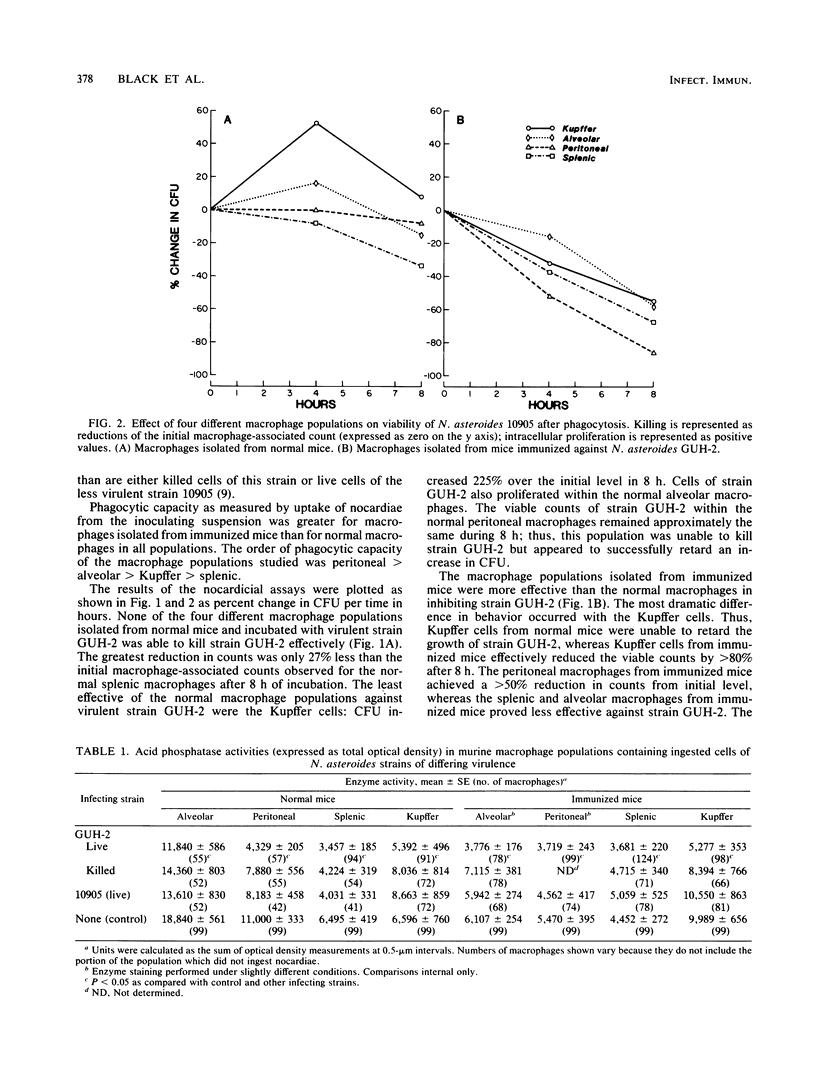
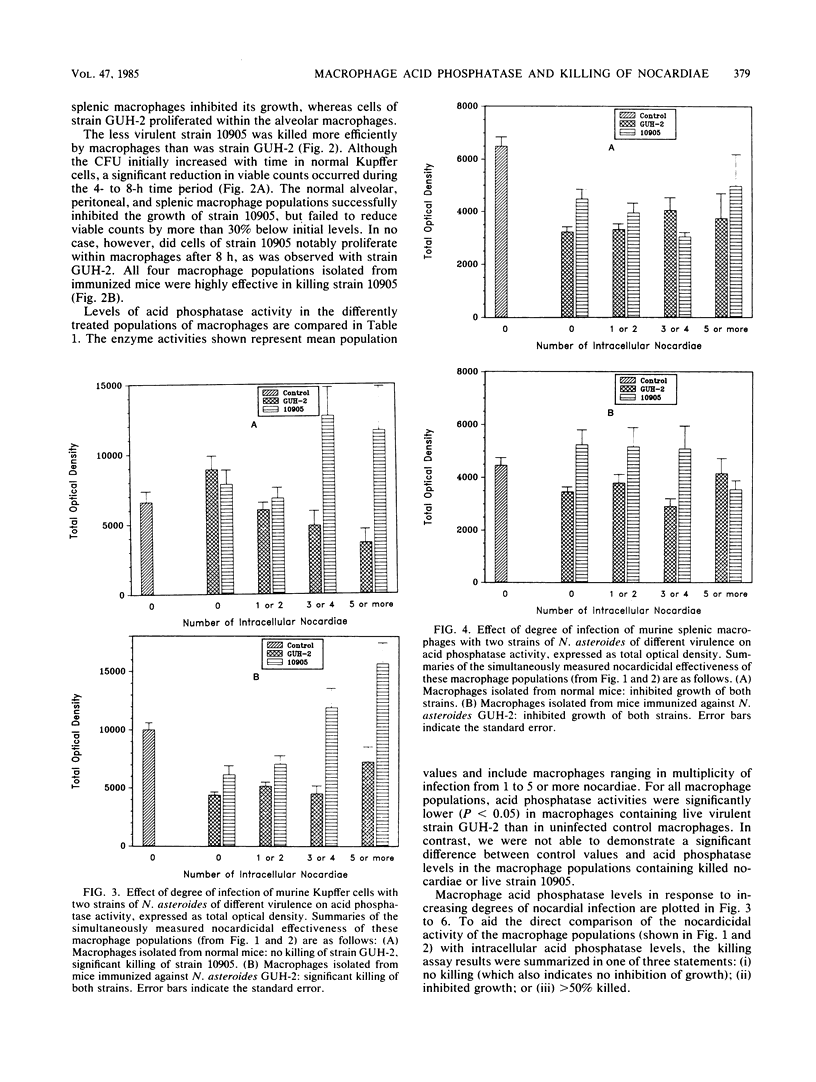
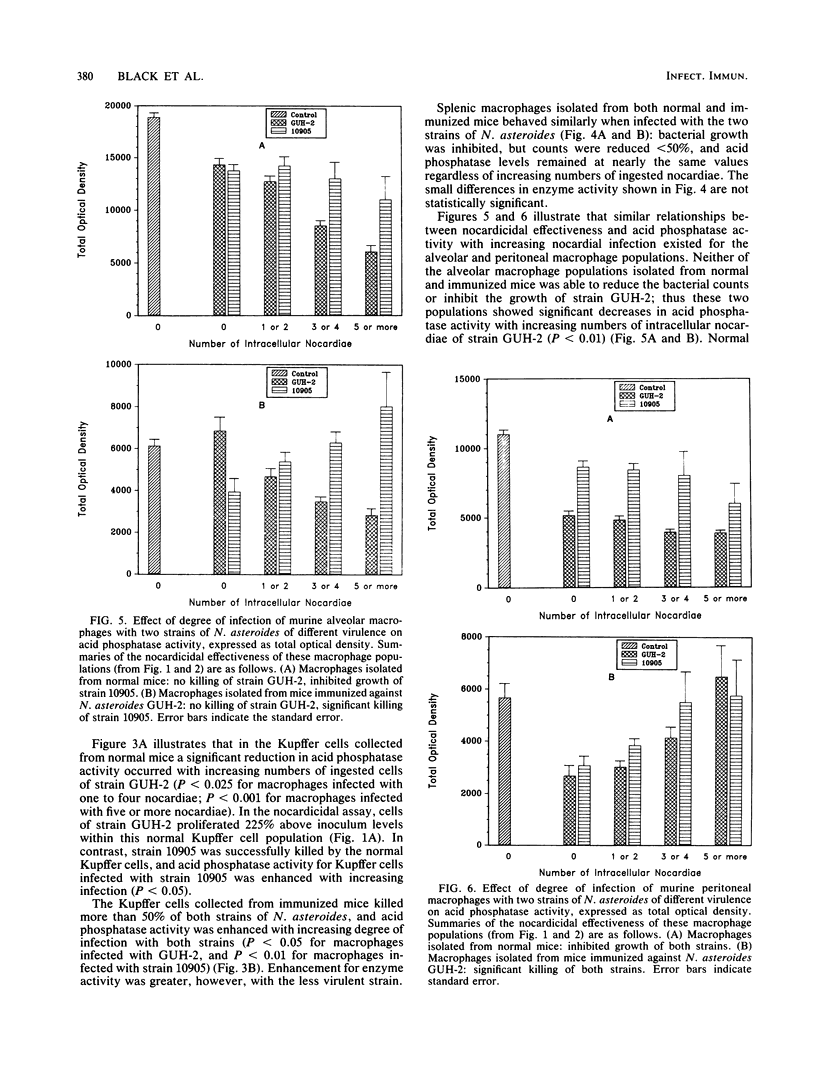
It has been reported that the activity of lysosomal acid phosphatase decreases inversely with numbers of ingested virulent Nocardia spp. in normal murine peritoneal and alveolar macrophages. These studies suggested that this relationship correlated with the effectiveness of these macrophage populations in killing Nocardia asteroides. Experiments were designed to determine if acid phosphatase activity is affected by infection with N. asteroides in four different macrophage populations isolated from normal and nocardia-immunized mice. Macrophages were also tested simultaneously for their ability to kill N. asteroides. Peritoneal, alveolar, and splenic macrophages and Kupffer cells were infected in vitro with strains of N. asteroides of differing virulence. Uptake and killing assays were performed. Acid phosphatase levels and numbers of intracellular nocardiae were quantitated in the same macrophages, using a computer-assisted cytophotometry system. Acid phosphatase activity decreased inversely with numbers of intracellular nocardiae in macrophages that could not kill or inhibit this pathogen. Acid phosphatase activity was not significantly changed in macrophages that inhibited growth of, but did not kill, N. asteroides, whereas activity was increased or enhanced in macrophages that killed most of the ingested nocardiae. The order of nocardicidal effectiveness (and resistance to enzyme activity reduction with infection) for normal macrophages was splenic greater than peritoneal greater than alveolar greater than Kupffer. In contrast, the order of these two parameters for macrophages isolated from immunized mice was Kupffer greater than peritoneal greater than alveolar greater than splenic. These results demonstrate that lysosomal acid phosphatase activity is an effective marker of the ability of macrophages to inhibit growth of and kill N. asteroides and that macrophages isolated from different anatomical sites differ functionally from each other with respect to nocardicidal and acid phosphatase activities.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L., Burnside J., Edwards B., Causey W. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976 Sep;134(3):286–289. doi: 10.1093/infdis/134.3.286. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Ahmed A., Scates S. M., Deem R. Response of CBA/N x DBA2/F1 mice to Nocardia asteroides. Infect Immun. 1982 Jan;35(1):111–116. doi: 10.1128/iai.35.1.111-116.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Scates S. S., Ohsugi Y. Immunobiology of germfree mice infected with Nocardia asteroides. Infect Immun. 1980 Aug;29(2):733–743. doi: 10.1128/iai.29.2.733-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Goldstein E., Gershwin M. E., Maslan S., Lippert W. Lung response to congenitally athymic (nude), heterozygous, and Swiss Webster mice to aerogenic and intranasal infection by Nocardia asteroides. Infect Immun. 1978 Dec;22(3):867–877. doi: 10.1128/iai.22.3.867-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Interaction of Nocardia asteroides at different phases of growth with in vitro-maintained macrophages obtained from the lungs of normal and immunized rabbits. Infect Immun. 1979 Oct;26(1):355–361. doi: 10.1128/iai.26.1.355-361.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect Immun. 1977 Jun;16(3):995–1004. doi: 10.1128/iai.16.3.995-1004.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Virulence of Nocardia asteroides during its growth cycle. Infect Immun. 1978 Apr;20(1):290–295. doi: 10.1128/iai.20.1.290-295.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. M., Beaman B. L., Donovan R. M., Goldstein E. Effect of virulent and less virulent strains of Nocardia asteroides on acid-phosphatase activity in alveolar and peritoneal macrophages maintained in vitro. J Infect Dis. 1983 Jul;148(1):117–124. doi: 10.1093/infdis/148.1.117. [DOI] [PubMed] [Google Scholar]
- Bourgeois L., Beaman B. L. Probable L-forms of Nocardia asteroides induced in cultured mouse peritoneal macrophages. Infect Immun. 1974 Mar;9(3):576–590. doi: 10.1128/iai.9.3.576-590.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Niederbuhl C. J., Campbell S. G. Bactericidal activity of alveolar and peritoneal macrophages exposed in vitro to three strains of Pasteurella multocida. Infect Immun. 1983 Feb;39(2):779–784. doi: 10.1128/iai.39.2.779-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of Nocardia asteroides with rabbit alveolar macrophages: association of virulence, viability, ultrastructural damage, and phagosome-lysosome fusion. Infect Immun. 1980 May;28(2):610–619. doi: 10.1128/iai.28.2.610-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN B. A., KROSS D. J., CIRCO R. Host-parasite relationships in brucellosis. II. Destruction of macrophage cultures by Brucella of different virulence. J. J Infect Dis. 1961 May-Jun;108:333–338. doi: 10.1093/infdis/108.3.333. [DOI] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Remington J. S. Effects of activated macrophages on Nacardia asteroides. Infect Immun. 1980 Feb;27(2):643–649. doi: 10.1128/iai.27.2.643-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Bartlema H. C., van der Ploeg M., van Duijn P., van der Stap J. G., Lippert W. Effect of ozone on lysosomal enzymes of alveolar macrophages engaged in phagocytosis and killing of inhaled Staphylococcus aureus. J Infect Dis. 1978 Sep;138(3):299–311. doi: 10.1093/infdis/138.3.299. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lojda Z., Van der Ploeg M., Van Duijn P. Phosphates of the naphthol AS series in the quantitative determination of alkaline and acid phosphatase activities "in situ" studied in polyacrylamide membrane model systems and by cytospectrophotometry. Histochemie. 1967;11(1):13–32. doi: 10.1007/BF00326609. [DOI] [PubMed] [Google Scholar]
- Palmer D. L., Harvey R. L., Wheeler J. K. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine (Baltimore) 1974 Sep;53(5):391–401. doi: 10.1097/00005792-197409000-00005. [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. I., Davis C. E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983 Dec;42(3):1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. W., Roth R., Loose L. D. A rapid, inexpensive and easily quantified assay for phagocytosis and microbicidal activity of macrophages and neutrophils. J Immunol Methods. 1979;29(3):221–226. doi: 10.1016/0022-1759(79)90309-0. [DOI] [PubMed] [Google Scholar]
- Simpson G. L., Stinson E. B., Egger M. J., Remington J. S. Nocardial infections in the immunocompromised host: A detailed study in a defined population. Rev Infect Dis. 1981 May-Jun;3(3):492–507. doi: 10.1093/clinids/3.3.492. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg M., Van den Broek K., Smeulders A. W., Vossepoel A. M., Van Duijn P. HIDACSYS: computer programs for interactive scanning cytophotometry. Histochemistry. 1977 Dec 28;54(4):273–288. doi: 10.1007/BF00508271. [DOI] [PubMed] [Google Scholar]
- Wilder M. S., Edberg J. C. Interaction of virulent and avirulent Listeria monocytogenes with cultured mouse peritoneal macrophages. Infect Immun. 1973 Mar;7(3):409–415. doi: 10.1128/iai.7.3.409-415.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg M., van Duijn P., Ploem J. S. High-resolution scanning-densitometry of photographic negatives of human metaphase chromosomes. II. Feulgen-DNA measurements. Histochemistry. 1974;42(1):31–46. doi: 10.1007/BF00498477. [DOI] [PubMed] [Google Scholar]


