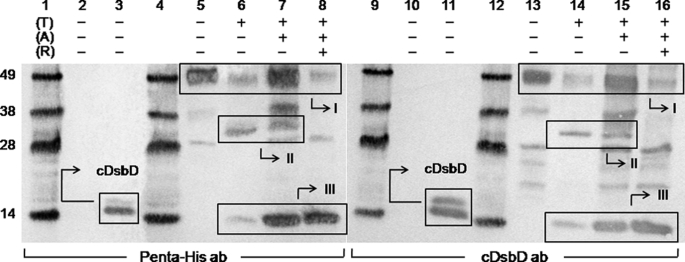
FIGURE 2.
Western blots of purified full-length C464A thrombin-cleavable DsbD before and after thrombin cleavage. Penta-His HRP-conjugated antibody was used in lanes 1-8 and goat antiserum raised against cDsbD of E. coli DsbD was used in lanes 9-16. Lanes 1, 4, 9, and 12 show molecular mass markers (from the top these are 49, 38, 28, and 14 kDa); lanes 2 and 10 show the negative control (nDsbD with no affinity tag); lanes 3 and 11 show the positive control (cDsbD bearing a C-terminal His6 tag). C464A thrombin-cleavable DsbD is shown in lanes 5 and 13 before alkylation of free thiols (A) and thrombin cleavage (T), in lanes 6 and 14 without alkylation and after thrombin cleavage, in lanes 7 and 15 after alkylation and thrombin cleavage, and in lanes 8 and 16 after alkylation and thrombin cleavage in the presence of reductant (R). The cDsbD band can be seen at ∼14 kDa in lanes 3 and 11, and in 6-8 and 14-16 in Box III (this construct runs as a diffuse double band on SDS-PAGE because its PelB signal sequence, which targets the protein to the periplasm, is cleaved inefficiently by the signal peptidase, leaving a large fraction of the protein uncleaved (confirmed by mass spectrometry and N-terminal sequencing)). The uncleaved C464A thrombin-cleavable DsbD band can be seen at ∼49 kDa (the actual mass of the protein is 60.8 kDa; a difference between the two masses is common for membrane proteins) in lanes 5-8 and 13-16 (box I) along with another band at ∼28 kDa that is a contaminant commonly seen after the purification of this protein. The nDsbD-cDsbD mixed disulfide band can be seen at ∼32 kDa in lanes 6, 7, 14, and 15 (box II) and it disappears in the presence of reductant in lanes 8 and 16. 2-3 μg of total protein were loaded in each lane.