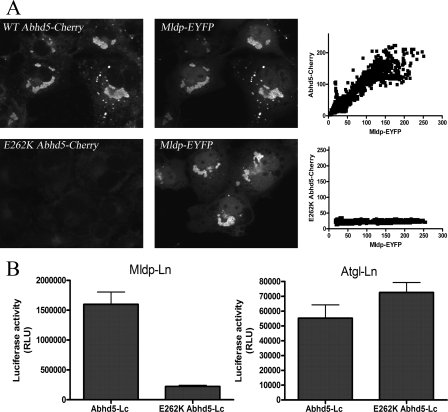
FIGURE 6.
E262K mutation of Abhd5 disrupts its interaction with Mldp. A, COS-7 cells were transfected with Mldp-EYFP and treated with oleic acid overnight. Cells were fixed and permeabilized and incubated with 293T lysates containing equal concentrations of wild type or E262K Abhd5-Cherry. Binding of wild type Abhd5 was in direct proportion to Mldp concentration, as determined by line scan analysis of fluorescence (right panel). No specific binding to Mldp was detected for the E262K mutant. B, the interaction of wild type and mutant Abhd5 with Mldp and Atgl was assessed by luciferase complementation assay. E262K mutation disrupted binding to Mldp but not to Atgl. Shown is a representative experiment performed in quadruplicate. The experiment was performed three times with similar results.