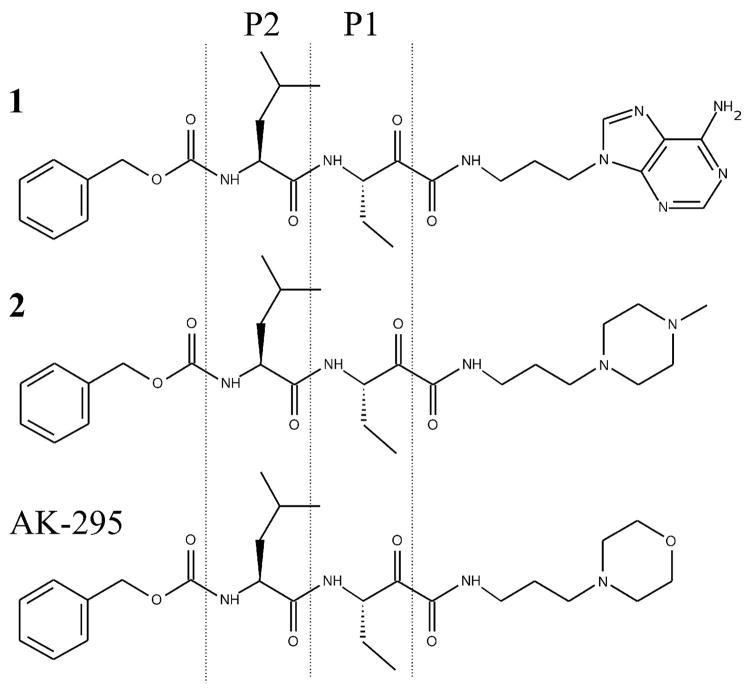
Figure 1.
Chemical structures of 1 and 2, the two α-ketoamide calpain inhibitors used in this study. Both compounds contain a P1 α-aminobutanoic acid and P2 leucine residue, but the primed side extension of 1 ends with adenine ring, while that of 2 is terminated by a piperazine ring. The structure of the commercially available calpain inhibitor AK-295 is also shown for comparison.