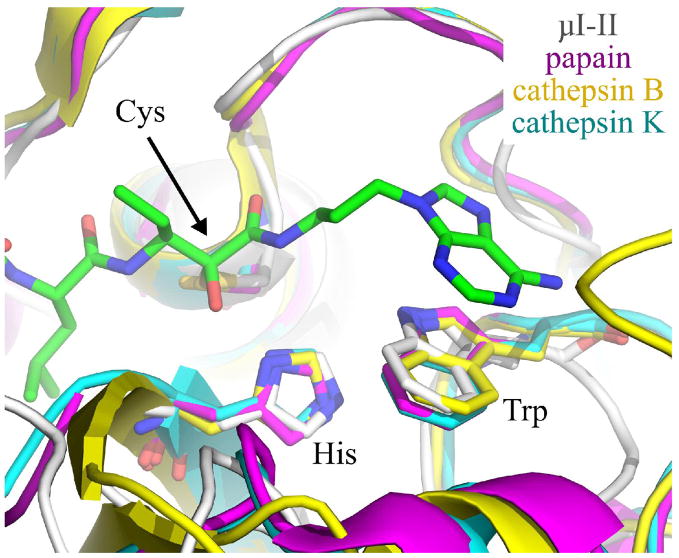
Figure 6.
Overlay of Trp298 in the μI–II-1 complex (μI–II in white, 1 in green) with the corresponding tryptophan in papain (magenta), cathepsin B (yellow) and cathepsin K (cyan). This overlay was achieved by first aligning three catalytic site residues (cysteine, histidine, tryptophan) of papain, cathepsin B and cathepsin K with those of the μI–II-1 structure. Inhibitor 1 and the overlaid cysteine, histidine and tryptophan residues are shown as sticks. The relatively similar alignments between the tryptophans suggests that aromatic stacking, by itself, will be insufficient to specifically target an inhibitor into the calpain active site. The PDB entries for the cysteine proteases are as follows: papain bound to leupeptin (1POP), human liver cathepsin B (1HUC) and cathepsin K bound to E-64 (1ATK). Nitrogens are colored blue, while oxygens are red.